MedTech News
.................... by Andrew Celentano
3D-printed scaffold process offers hope for spinal cord injury recovery
For the first time, a research team at the University of Minnesota Twin Cities demonstrated a process that combines 3D printing, stem cell biology, and lab-grown tissues for spinal cord injury recovery.
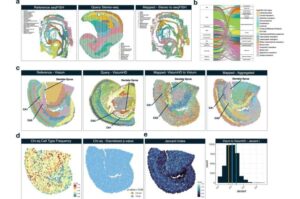
Cell-mapping tool provides insightful multi-layered view of cancer behavior
Researchers at VCU Massey Comprehensive Cancer Center have developed a new computational tool called Vesalius, which could help clinicians understand the complex relationships between cancer cells and their surrounding cells, leading to potential discoveries regarding the development of hard-to-treat cancers.
Wearable sweat sensor can detect responses to physical, emotional and pharmacological stress
Most people are well aware of the effects of chronic stress in the modern world. While some stress can be a good thing, like the type of stress your body feels during an intense workout, prolonged or chronic stress can lead to a myriad of health problems, including anxiety, heart disease, and inflammation. And, at a larger scale, the high prevalence of chronic stress in the population increases the burden on public health systems.

A new tool to track infant development, starting at just 16 days old
Northwestern unveils NIH Baby Toolbox, the newest national standard to assess infants’ cognitive, emotional growth

Abbott wins CE mark for Esprit drug-eluting, dissolving scaffold
Abbott (NYSE: ABT)+
announced today that it received CE mark for its Esprit BTK everolimus-eluting resorbable scaffold system.
Key genes that act as a brake on blood cancer growth reveal potential treatment targets
Australian researchers have used an innovative genome-wide screening approach to identify genes, and their encoded proteins, that play critical roles in the prevention of lymphoma development, revealing new potential treatment targets for these blood cancers.

Scientists discover new ‘3D genome organizer’ linked to fertility and cancer
A research team at Kyoto University has discovered STAG3-cohesin, a new mitotic cohesin complex that helps establish the unique DNA architecture of spermatogonial stem cells (SSCs), the stem cells that give rise to sperm.
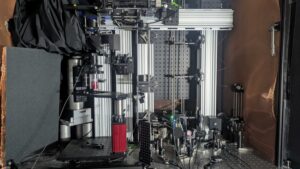
Imaging tech promises deepest looks yet into living brain tissue at single-cell resolution
By combining several cutting-edge imaging technologies, a new microscope system could enable unprecedentedly deep and precise visualization of metabolic and neuronal activity, potentially even in humans.
