MedTech News
.................... by Andrew Celentano
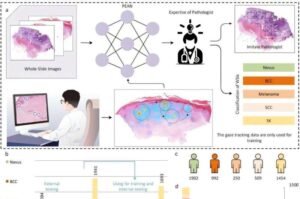
Scientists train deep-learning models to scrutinize biopsies like a human pathologist
In the Age of AI, many health care providers dream of a digital assistant, unencumbered by fatigue, workload, burnout or hunger, that could provide a quick second opinion for medical decisions, including diagnoses, treatment plans and prescriptions.
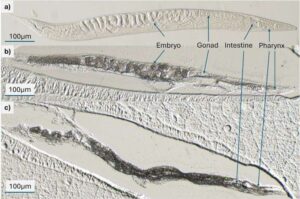
Mapping fat: How microfluidics and mass spectrometry reveal lipid landscapes
Understanding how fat molecules are distributed and function in living organisms is key to uncovering mechanisms of aging, disease, and metabolism.
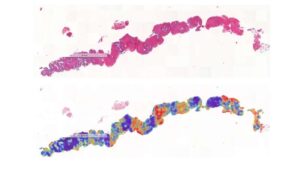
AI detects early prostate cancer in more than 80% of samples missed by pathologists
Men assessed as healthy after a pathologist analyzes their tissue sample may still have an early form of prostate cancer. Using AI, researchers at Uppsala University have been able to find subtle tissue changes that allow the cancer to be detected long before it becomes visible to the human eye.
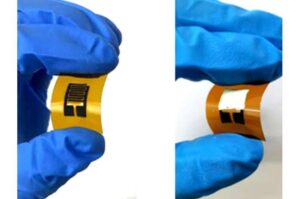
Diagnosing diabetes may soon be as easy as breathing into a bag
In the U.S., one in five of the 37 million adults who has diabetes doesn’t know it. Current methods of diagnosing diabetes and prediabetes usually require a visit to a doctor’s office or lab work, both of which can be expensive and time-consuming. Now, diagnosing diabetes and prediabetes may be as simple as breathing.
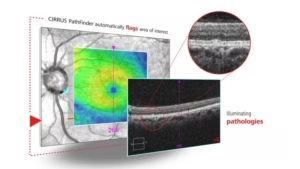
Zeiss’ AI-driven tool gets CE mark for OCT scans
The CIRRUS PathFinder tool is available via licensing in the new software update.
EPO grants patent for Autonomix Medical’s catheter technology
The patent covers a broad spectrum of applications, including arterial and renal mapping.
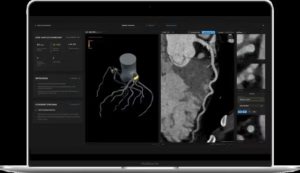
FDA clearance of Salix® Coronary Plaque module
PERTH, Australia, Aug. 21, 2025 /PRNewswire/ — Artrya Limited (ASX: AYA) (Artrya or the Company), a medical technology company commercialising its Salix® AI-powered cloud platform, for the near real time, point of care assessment and management of coronary artery disease, is pleased to announce it has received 510(k) clearance from the U.S. Food and Drug Administration (the FDA) for Artrya’s proprietary, Salix® Coronary Plaque module.
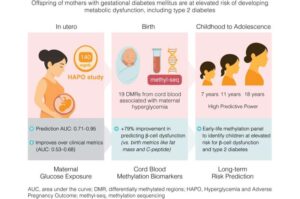
A simple test could predict a newborn’s risk of developing type 2 diabetes
A genetic test of cord blood at birth may hold the key to predicting a child’s future risk of developing type 2 diabetes, according to exciting new research from Australia and Hong Kong.
