MedTech News
.................... by Andrew Celentano

Under-the-skin electrode allows for real-world epilepsy tracking
New research from the Institute of Psychiatry, Psychology & Neuroscience (IoPPN) at King’s College London in partnership with the Mayo Clinic and UNEEG medical, has found that an electronic device placed under the scalp is an effective and feasible means of accurately tracking epilepsy.
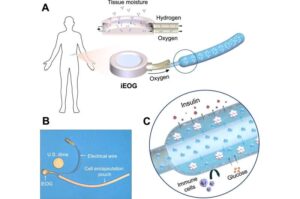
Implant treats type 1 diabetes by oxygenating insulin-producing cells
Cornell researchers have developed an implant system that can treat type 1 diabetes by supplying extra oxygen to densely packed insulin-secreting cells, without the need for immunosuppression.
Dräger Atlan anesthesia workstations obtain Authority to Operate (ATO) under Risk Management Framework
TELFORD, Pa., Aug. 11, 2025 /PRNewswire/ — Today Dräger, an international leader in medical and safety technologies, announced that the company’s Atlan A350/A350 XL series of anesthesia workstations received Authority to Operate (ATO) certification under the Department of Defense’s (DoD) Risk Management Framework (RMF) eMASS #4060.
Dana-Farber develops blood test for multiple myeloma diagnosis
The method assesses growth rates of the tumour and detects gene patterns that can forecast outcomes of the patient.

FDA approves Nyxoah’s Genio system for obstructive sleep apnoea
The system is compatible with 1.5T and 3T magnetic resonance imaging scans and eliminates the need for an implanted battery.
EDX Medical develops rapid pneumonia test for hospital patients
EDX claims the test, which is intended for use in the ICU, provides results for guiding treatment decisions within 60 seconds.
FDA approves expansion to Orchestra BioMed pacemaker trial
Orchestra BioMed (Nasdaq:OBIO) announced today that it began the rollout of a protocol update for its BACKBEAT study.

Nyxoah wins FDA nod for sleep apnea neuromod system
Nyxoah (Nasdaq:NYXH) announced today that it received FDA approval for its Genio neuromodulation device for treating sleep apnea.
