MedTech News
.................... by Andrew Celentano

Edgepark expands diabetes offerings with two new product innovations
TWINSBURG, Ohio, July 15, 2025 /PRNewswire/ — Edgepark has recently expanded its portfolio to include the latest innovations from diabetes manufacturers, including the first-ever FDA-cleared yearlong continuous glucose monitor (CGM) system, and a glucose biosensor that can be purchased without a prescription.

Research shows early cancer cells can be rapidly detected through electronic semiconductors
CLEVELAND, July 15, 2025 /PRNewswire/ — IdentifySensors Biologics, a leader in digital diagnostic technologies, has demonstrated that its electronic biosensors used to detect pathogens can also detect cancer cells.

Treating a Viral Infection in Cats May Solve the Mystery of Long COVID
Learn about the disease in cats that shows striking similarities to long COVID and the treatment that can restore feline immune dysfunction.

Lung Scan Technology Reveals Hidden Secrets of Airway Disease
LOS ANGELES, July 15, 2025 /PRNewswire/ — Researchers at Vanderbilt University, Johns Hopkins University, University of Miami, and The Alfred Hospital studied the lungs of subjects including Veterans using technology developed by 4DMedical called X-ray Velocimetry Lung Ventilation Analysis Software (XV LVAS®).
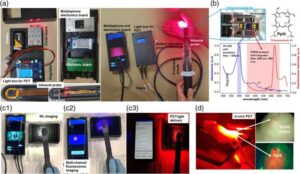
Handheld device enables imaging and treatment of oral cancer in low-resource settings
Oral cancer is a growing public health concern, particularly in South Asia, where it affects tens of thousands each year. In India alone, oral cancer accounts for 40% of all cancers, largely driven by the widespread use of tobacco-based products like gutka.
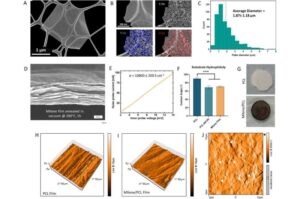
Researchers develop 3D-printed implant to help repair spinal cord injuries
A research team at RCSI University of Medicine and Health Sciences has developed a 3D printed implant to deliver electrical stimulation to injured areas of the spinal cord, offering a potential new route to repair nerve damage. Details of the 3D-printed implant and how it performs in lab experiments have been published in the journal Advanced Science.
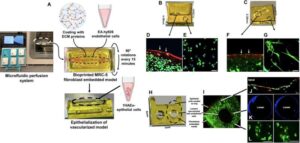
Researchers create 3D-printed living lung tissue
UBC Okanagan researchers have developed a 3D bio-printed model that closely mimics the complexity of natural lung tissue, an innovation that could transform how scientists study lung disease and develop new treatments.

AI-based tool can ‘measure’ cancer aggressiveness and paves the way for new therapies
As cancer cases have increased worldwide, the disease has become more complex, presenting challenges to scientific advances in diagnosis and treatment. In this context, artificial intelligence (AI) has emerged as a valuable tool for predicting and detecting cases.
