MedTech News
.................... by Andrew Celentano
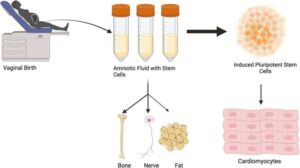
Amniotic stem cells can be collected from vaginal fluid rather than more invasive techniques
Researchers at the University of Colorado Anschutz Medical Campus have discovered that amniotic fluid stem cells can be safely collected from vaginal fluid after childbirth rather than relying on more invasive methods that can pose some risk to the mother and fetus.
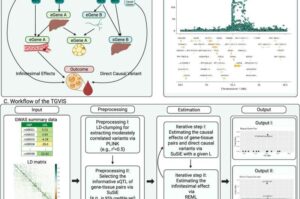
New gene tool leads to better treatments for complex diseases
Genetic changes can signal evidence of disease, but pinpointing which genes and what’s changed can be difficult.
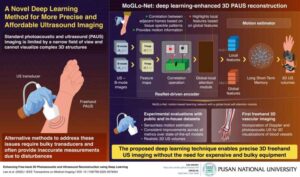
New deep learning model enhances handheld 3D medical imaging
Ultrasound (US) imaging is a widely employed diagnostic tool used for real-time imaging of various organs and tissues using ultrasonic sound waves. The waves are sent into the body, and images are created based on how the waves reflect off internal tissues and organs. It is used for guiding many medical procedures, including biopsies and injections, and is important for dynamic monitoring of blood vessels.
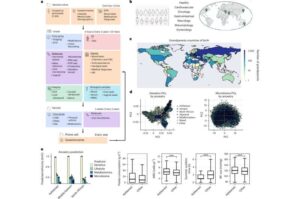
AI-powered ‘digital twin’ created to predict personal health outcomes
Recently, researchers from Prof. Eran Segal’s laboratory at the Weizmann Institute of Science have harnessed artificial intelligence to create a personalized “digital twin” that allows them to detect a risk of developing diseases, initiate preventive treatment and even run simulations to predict which treatment will be most effective.
Medtronic gets CE mark for robot-driven vessel-sealing device
Medtronic said its market-leading LigaSure technology can now be used with its Hugo soft tissue robotic surgery system in Europe.

HistoSonics Secures Expanded Insurance Coverage for Liver Tumor Therapy
Non-Invasive Histotripsy Treatment Now Accessible to 7 Million Blue Cross Blue Shield Highmark Members Across U.S.

Erbe Launches FiAPC® plus to Elevate Argon Plasma Coagulation Precision
Next-Generation Technology Enhances Control, Safety, and Efficiency in Flexible Endoscopic Procedures
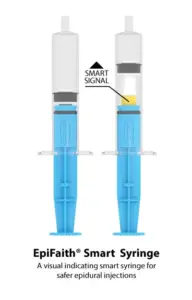
Mercury Medical Partners with Flat Medical to Launch EpiFaith® Smart Syringe in the U.S.
Innovative Partnership Aims to Enhance Epidural Safety with Real-Time Pressure Sensing Technology for Improved Patient Outcomes
