MedTech News
.................... by Andrew Celentano
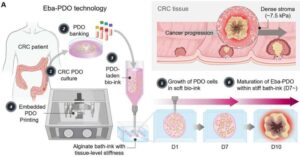
Bioprinted organoids capture tumor features and predict prognosis based on shape
A joint research team affiliated with UNIST has developed a 3D-printed artificial tumor tissue capable of replicating the in vivo conditions of patient-derived cancer cells. This innovative model not only simulates the tumor microenvironment but also integrates artificial intelligence (AI) technology that can predict patient prognosis solely from images of tumor growth.

Scientists film the heart forming in 3D earlier than ever before
Researchers at UCL and the Francis Crick Institute have, for the first time, identified the origin of cardiac cells using 3D images of a heart forming in real-time, inside a living mouse embryo.
Tivic adds new vagus nerve stim patent
Tivic Health Systems (Nasdaq:TIVC) filed a patent application for its vagus nerve stimulation (VNS) system.
Tandem Diabetes Care subsidiary earns new FDA clearance for insulin infusion set
A subsidiary of Tandem Diabetes Care (Nasdaq:TNDM) recently picked up FDA clearance for infusion technology to support insulin delivery
Better than stitches: Researchers develop biocompatible patch for soft organ injuries
University of California, Los Angeles and University of California, San Diego researchers developed an injectable sealant for rapid hemostasis and tissue adhesion in soft, elastic organs.

A more realistic look at DNA in action
Researchers find that DNA behaves differently when crowded by molecules, as in a cell

True Indicating’s CSPN-15 Steam Integrator Earns FDA Approval for Advanced Sterilization Assurance
High-Precision Type 5 Indicator Enhances Patient Safety by Validating Steam Sterilization Cycles in Clinical Settings

Curaleaf International and Jupiter Research Secure EU Certification for Pioneering Handheld Liquid Inhalation Device
Partnership Pioneers the First-Ever Certified Handheld Liquid Inhalation Device for Medical Use in Europe, Expanding Access to Innovative Treatment Options
