MedTech News
.................... by Andrew Celentano

FDA Approves Teal Health’s Teal Wand™–The First and Only At-Home Self-Collection Device for Cervical Cancer Screening, Introducing a Comfortable Alternative to In-Person Screening
Women can now collect their own sample from the privacy of home, no speculum required, and mail it to a certified lab to be tested on the same test as the doctor’s office, with the same accuracy.
Zydus Gains USFDA Approval for Generic Copaxone® Injection
Final approval clears the way for Zydus to market Glatiramer Acetate, expanding access to a key treatment for multiple sclerosis patients in the U.S.
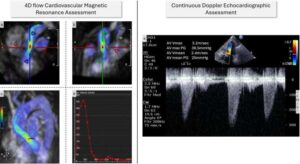
Improving the diagnosis of a common heart problem with new MRI technology
University of East Anglia scientists have developed cutting-edge MRI technology to diagnose a common heart problem more quickly and accurately than ever before.
Early trials of smallpox vaccine against mpox show positive safety and efficacy results
In recent years, the world has seen a surge in new and deadly infectious diseases, posing a major threat to global health. Outbreaks of COVID-19, H1N1 (swine flu), Ebola, Zika, and monkeypox are a stark reminder of our vulnerability.

Wireless device enables precise activation of light-sensitive pain drugs in animal study
Photoactivable drugs are activated when irradiated by a beam of light—via an optical fiber—thus generating a controlled and local therapeutic effect on target tissues. Now, a scientific team has pioneered a new breakthrough in the field of photopharmacology with the design of the first wireless device capable of remotely activating a photoactivable drug and causing it to have therapeutic effects on specific organs.
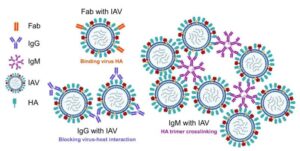
Engineered antibody nasal spray protects mice from deadly influenza A infection
Scientists have engineered a monoclonal antibody that can protect mice from a lethal dose of influenza A, a new study shows. The new molecule combines the specificity of a mature flu fighter with the broad binding capacity of a more general immune system defender.

Lab-on-a-chip devices offer home tests for stress and cardiac issues
University of Cincinnati engineers have created a new device to help doctors diagnose depression and anxiety.
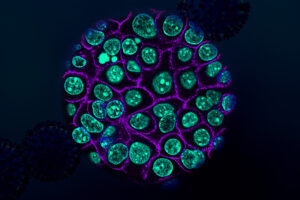
Biologists identify targets for new pancreatic cancer treatments
Their study yielded hundreds of “cryptic” peptides that are found only on pancreatic tumor cells and could be targeted by vaccines or engineered T cells.
