MedTech News
.................... by Andrew Celentano

Non-invasive sensor measures intracranial pressure more accurately, aiding early intervention
A technology developed by the Brazilian company brain4care has been shown to be able to measure absolute values of intracranial pressure (ICP) more accurately than existing non-invasive methods. This is the result of a study published in the journal npj Digital Medicine by researchers from the University of São Paulo in Brazil, the University of Cambridge in the United Kingdom, Emory University in the United States, and the company itself.
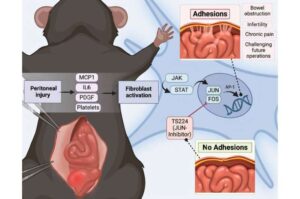
An easy-to-apply gel prevents abdominal adhesions following surgery in animal study
Surgical adhesions—common, sometimes life-threatening complications that arise after open or laparoscopic abdominal surgery—can be prevented in mice and pigs by a gel impregnated with a molecule that blocks a key signaling pathway in the formation of scar tissue.
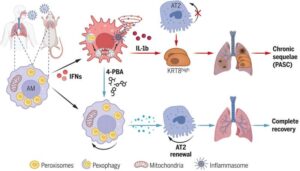
COVID-19 discovery opens door to new treatments for chronic lung problems
University of Virginia School of Medicine scientists have discovered how severe COVID-19 can destroy immune cells’ ability to repair the lungs, helping explain the lingering effects of long COVID. The findings suggest a new treatment approach for long COVID as well as other conditions, both short-term and chronic, caused by respiratory infections such as the flu.
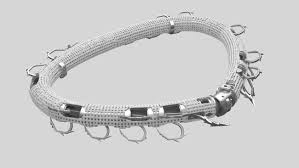
Valcare Medical Receives FDA Approval to Initiate Early Feasibility Study for its Novel AMEND™ Trans-Septal System
HERZLIYA, Israel and WILMINGTON, Del., March 13, 2025 /PRNewswire/ — Valcare Medical Inc., a leading innovator in transcatheter-based mitral solutions, today announced the U.S. Food and Drug Administration (FDA) has approved the AMEND™ Trans-Septal System for investigational device exemption (IDE) application to commence an Early Feasibility Study (EFS).

Vave Health Introduces World’s First Wireless, Handheld, Whole-Body Ultrasound with a Single PZT Transducer
SAN JOSE, Calif., March 13, 2025 /PRNewswire/ — Vave Health proudly announces the launch of its Universal Wireless Probe. This simple, innovative device is designed to enhance efficiency, accuracy, and diagnostics in a variety of clinical and educational settings. Its combination of linear and phased imaging technology provides high quality, full-body imaging with no ongoing subscription fees or hidden costs.

Vivani reports first human GLP-1 implant for weight loss in adults
Vivani Medical (Nasdaq:VANI) announced today that it successfully administered the first GLP-1 implant in its LIBERATE-1 clinical trial.

Johnson & Johnson MedTech wins FDA nod for enhanced robotic-assisted bronchoscopy
Johnson & Johnson MedTech (NYSE: JNJ)+
announced today that the FDA cleared its Monarch Quest technology for robotic-assisted bronchoscopy.
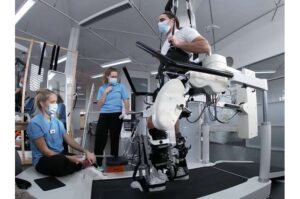
Robotics and spinal stimulation restore movement in paralysis
Spinal cord injuries are life-altering, often leaving individuals with severe mobility impairments. While rehabilitation robotics—devices that guide movement during therapy—have improved training for those with spinal cord injuries, their effectiveness remains limited. Without active muscle engagement, robotic-assisted movement alone does not sufficiently retrain the nervous system.
