MedTech News
.................... by Andrew Celentano
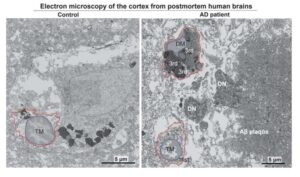
Alzheimer’s progression tied to stress-induced microglial lipid release
Researchers with the Advanced Science Research Center at the CUNY Graduate Center (CUNY ASRC) have unveiled a critical mechanism that links cellular stress in the brain to the progression of Alzheimer’s disease (AD).
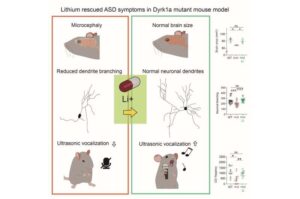
Lithium shows promise in treating autism-related symptoms in mouse study
A discovery has highlighted lithium—a drug long used to treat bipolar disorder and depression—as a potential therapy for autism spectrum disorder (ASD). This research, conducted by a team at the Center for Synaptic Brain Dysfunctions within the Institute for Basic Science (IBS) led by Director Kim Eunjoon, reveals that lithium can restore brain function and alleviate behavioral symptoms in animal models of ASD caused by mutations in the Dyrk1a gene.
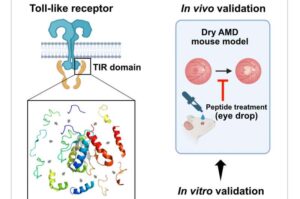
Peptide-based eye drops show promise in treating age-related macular degeneration
Age-related macular degeneration (AMD) is the leading cause of vision loss in individuals over 65, characterized by abnormal changes in the macular, resulting in reduced vision and distorted objects. Dry AMD accounts for 90% of all AMD cases, with relatively mild vision impairment; however, approximately 30% progress to the severe vision loss associated with wet AMD within 10 years.
Unclogging the immune system: Scientists use immunotherapy to remove aging cell buildup
Whenever a sink overflows, the flooding is usually caused by a blockage that has built up in the drains. Similarly, as we age, our bodies are flooded by aging, or senescent cells, which have stopped dividing but, instead of dying, remain active and build up in body tissues. Recent studies have shown that getting rid of these cells might delay age-related diseases, reduce inflammation and extend lives. Despite the great potential, however, there is currently no drug that can target these cells directly and efficiently.
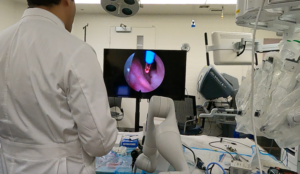
Andromeda Surgical announces first robot-assisted prostate procedures
Andromeda Surgical announced that a surgeon in Chile performed a robotic-assisted holmium laser enucleation of the prostate (HoLEP) with its platform.
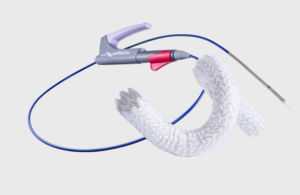
FDA approves Merit Medical’s cell-impermeable endoprosthesis
Merit Medical Systems (Nasdaq:MMSI) announced today that it received FDA premarket approval for its Wrapsody cell-impermeable endoprosthesis.
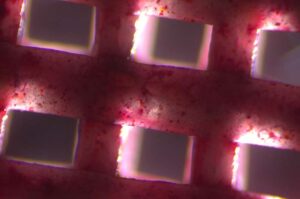
3D bioprinted scaffolds enhance bone healing through improved vascularization
Researchers from IBEC led by Oscar Castaño, senior researcher at the Biomaterials for Regenerative Therapies group, developed a novel approach, recently published in Biomaterials Advances.
Innovative red blood cell shape test promises better blood storage and transfusions
Scientists have developed a way of assessing the ability of red blood cells to deliver oxygen by measuring their shape. This test could improve specialist transplant and transfusion practice as well as blood banking. The research is published in eBioMedicine.
