MedTech News
.................... by Andrew Celentano

AngioDynamics Receives 510(k) Clearance for AlphaVac F1885 System in Treatment of PE
New Indication for Treatment of Pulmonary Embolism Enhances Device Utility in Critical Medical Scenarios

Nexalin Technology Awarded Key Patent Related to its Gen-3 HALO Clarity™, a Non-Invasive, Frequency-Based Deep-Brain Stimulation Device
This method of use patent award marks a major achievement for the Company and follows another recent patent covering our core technology.
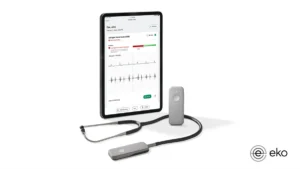
Eko wins FDA nod for AI to detect sign of heart failure using stethoscope
The clearance adds to the list of devices the FDA has authorized this year with AI algorithms to detect health conditions.
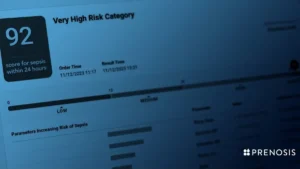
FDA grants de novo nod to AI tool for detecting sepsis
The CEO of Prenosis told MedTech Dive the company sees third-party validation as important, with the FDA having clarified that certain decision support tools should be regulated as medical devices.

Researchers 3D print key components for a point-of-care mass spectrometer
The low-cost hardware outperforms state-of-the-art versions and could someday enable an affordable, in-home device for health monitoring.

Haemonetics Receives FDA Clearance for New TEG® 6s Global Hemostasis – HN Cartridge
TEG testing provides critical information that can help physicians improve hemostasis management for their patients

WAT Medical wins FDA nod for OTC migraine preventative device
HeadaTerm 2 uses neuromodulation technology. It releases targeted electrical impulses that increase the pain tolerance of the wearer.
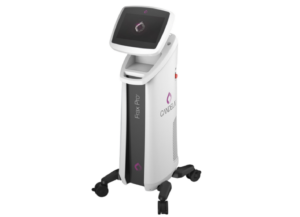
Venus Concept Announces Australian Regulatory Approval for Venus Versa Pro
The Venus Versa Pro combines the applicator of the Venus Viva MD with the Venus Versa system which are both approved in Australia and registered in Australian Register of Therapeutic Goods (ARTG).
