MedTech News
.................... by Andrew Celentano
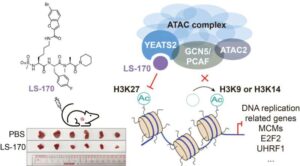
A new strategy to beat lung cancer: Chemists develop first-in-class inhibitor targeting a key epigenetic regulator
A research team has made a breakthrough in epigenetic drug discovery. The researchers have successfully developed a first-in-class chemical inhibitor that precisely and selectively targets the ATAC complex, a critical cellular “switch operator” that activates tumor-promoting genes, opening a novel therapeutic avenue for non-small cell lung cancer (NSCLC).

Gut microbiota can predict long-term complications of acute pancreatitis
A Europe-wide study led by the University Medical Center Göttingen (UMG) shows that the microbial composition of the gut, known as the gut microbiome, can predict long-term complications following severe acute pancreatitis.
Shingles vaccine linked to slower biological aging in older adults
Shingles vaccination not only protects against the disease but may also contribute to slower biological aging in older adults, according to a new USC Leonard Davis School of Gerontology study.

Blood test can identify cancer in patients with non-specific symptoms
A simple blood test can help detect cancer in patients with non-specific symptoms such as fatigue, pain or weight loss. This is according to a Swedish study from Karolinska Institutet, Danderyd Hospital and others, published in Nature Communications.
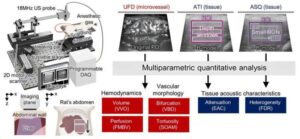
Steatotic liver disease precisely assessed using 3D ultrafast vascular ultrasound
Steatotic liver disease (commonly called fatty liver disease) progresses silently. Even in the absence of noticeable symptoms, changes begin to unfold inside the liver.
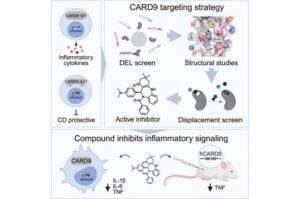
Small molecules could treat Crohn’s disease by mimicking a protective gene variant
An estimated 3 million Americans have an inflammatory bowel disease (IBD) such as Crohn’s disease or ulcerative colitis. But a lucky few individuals are far less likely to develop IBD because they have a rare variant of a gene called CARD9. This protective gene variant prevents the long-term digestive tract inflammation that can cause tissue damage and lead to disease.
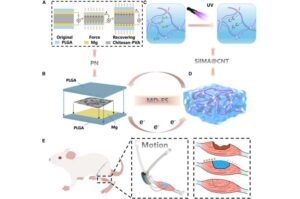
Researchers develop biodegradable, self-powered electrical stimulator for muscle repair
A research team led by Prof. Bai Shuo from the Institute of Process Engineering of the Chinese Academy of Sciences has pioneered a fully biodegradable, self-powered implantable electrical stimulation system designed to enhance muscle repair.

New coffee chemicals show promise for managing type 2 diabetes
Coffee may do more than boost energy. New research suggests that certain compounds found in roasted coffee beans could help slow how quickly sugar enters the bloodstream, a finding that could one day support new foods aimed at managing type 2 diabetes.
