MedTech News
.................... by Andrew Celentano

New toothpaste stops periodontal pathogens
Researchers at the Halle branch of the Fraunhofer Institute for Cell Therapy and Immunology IZI have identified a substance that selectively blocks harmful pathogens such as Porphyromonas gingivalis without affecting other bacteria.
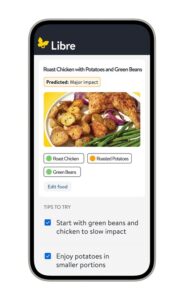
Abbott’s new Libre Assist app feature tackles a top need for people living with diabetes: in-the-moment food decisions
ABBOTT PARK, Ill., Jan. 5, 2026 /PRNewswire/ — Abbott (NYSE: ABT), a leading healthcare company, today unveiled Libre Assist,1 a groundbreaking feature within the Libre app5 designed to help the millions of people living with diabetes in the U.S. better understand how the foods they eat affect their glucose levels. 1,2 Unlike traditional food logging apps that only give feedback after a meal is logged, Libre Assist1 helps people make informed mealtime decisions before they eat. Abbott is launching the new technology during CES 2026 in Las Vegas.

Women’s Digital Health Challenge winners announced
Two pioneering, clinically-led digital app solutions have won the Ripple Women’s Digital Health Challenge – a global innovation programme, delivered by Cogniss in partnership with the Health Innovation Network (the Network) and Amazon Web Services (AWS).

Sumbu Unveils World’s First Dual-Vector Consumer Exoskeleton at CES 2026
LAS VEGAS, Jan. 3, 2026 /PRNewswire/ — Today at CES 2026, Sumbu announced its Exo-S3 line of dual-vector exoskeletons designed for the general public: the Exo-S3, Exo-S3 Pro and Exo-S3 Ultra. The series is the world’s first commercially available dual-vector exoskeleton designed to support human movement across real-world terrain. This release establishes a new market for lightweight, AI-powered wearable mobility products.

AI model predicts B cell reactivity to neoantigens for improved cancer vaccines
Professor Jung Kyoon Choi’s research team from the Department of Bio and Brain Engineering, in collaboration with Neogen Logic Co., Ltd., developed the new AI model to predict neoantigens.
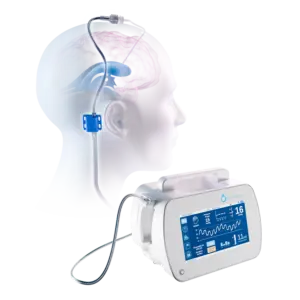
FDA Clears BrainSpace Intellidrop, an automated neuro device that addresses ICU nursing shortages and builds training data for Physical AI
SEATTLE, Jan. 2, 2026 /PRNewswire/ — BrainSpace, a medical technology company building automated solutions for neuro injury, illness and degeneration, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for Intellidrop.

HAI Solutions Receives FDA De Novo Grant for QIKCAP System
HAI Solutions, a leading innovator in ultraviolet (UVC) microbial reduction medical devices, announced that the U.S. Food and Drug Administration (FDA) has granted De Novo classification for its QIKCAP System. This marks the first and only FDA-Granted Ultraviolet light-based microbial reduction device for luer-activated valves, establishing a new Class II medical device category.

StimLabs Announces FDA Clearance of Theracor
Theracor is a sheet device derived from human umbilical cord extracellular matrix (ECM) intended to cover, protect, and provide a moist wound environment
