MedTech News
.................... by Andrew Celentano

Self-guided behavioral app helps children with epilepsy sleep earlier
An evidence-based web-app helped children with epilepsy to fall asleep on average 16.5 minutes earlier.
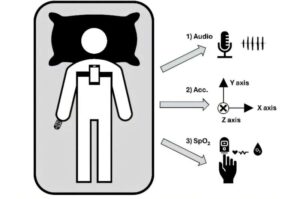
Mobile technology improves sleep apnea diagnosis after a stroke
Sleep apnea is a disorder characterized by repeated obstructions or collapses of the upper airway during sleep. These interruptions to breathing reduce the amount of oxygen in the blood. Stroke patients are at high risk, as they experience poorer sleep quality and have a higher prevalence of sleep disorders, such as insomnia and respiratory problems.
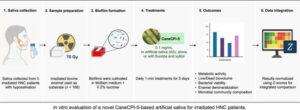
Artificial saliva with sugarcane protein shields teeth after cancer treatment
An artificial saliva in the form of a mouthwash, produced with the CANECPI-5 protein extracted from sugarcane and modified in a laboratory, can aid in treating teeth in patients with head and neck cancer. In these cases, radiotherapy very close to the mouth can destroy salivary glands and compromise saliva production, which is essential for controlling bacteria and disease.
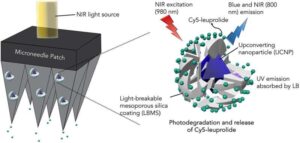
Light-triggered microneedle patch could make IVF hormone delivery painless and automated
A McGill University research team has developed a painless, automated way to deliver in vitro fertilization (IVF) hormones using a light-activated microneedle patch, an innovation that could ease one of the most stressful parts of fertility treatment and open new possibilities for other diseases that require frequent, time-sensitive injections.
Researchers uncover molecular roots of tissue scarring in inflammatory bowel disease
In earlier work, a team led by researchers at the Broad Institute and Mass General Brigham discovered a key cell type underlying fibrosis in inflammatory bowel disease (IBD). Now, in a new study published in Nature, the team has characterized the crosstalk between this and other types of cells that leads to fibrosis.
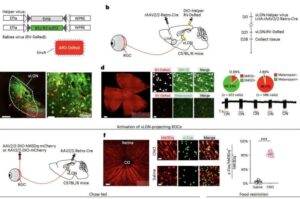
Bright light suppresses eating and weight gain in mice
Past research has found that exposure to bright lights and high levels of noise can alter both physiological processes and human behavior. For instance, an elevated or limited exposure to bright lights and noise has been found to influence people’s sleeping patterns, circadian rhythm, mood, metabolism, stress levels and mental performance.
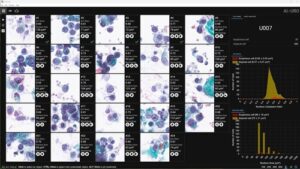
CorePlus Collaborates with AIxMED Providing 100% QC on Bladder Cancer Detection with AI
SAN JUAN, Puerto Rico, Jan. 7, 2026 /PRNewswire/ — CorePlus announced the implementation of AIxURO, a state-of-the-art software solution that utilizes artificial intelligence to significantly improve the accuracy and efficiency of bladder cancer detection. This innovative platform enhances traditional urine cytology analysis, providing faster, more precise diagnostics.

AI-generated sensors open new paths for early cancer detection
Nanoparticles coated with molecular sensors could be used to develop at-home tests for many types of cancer.
