MedTech News
.................... by Andrew Celentano
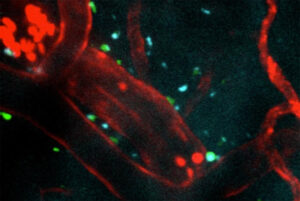
Post-stroke injection protects the brain in preclinical study
Nanomaterial crosses blood-brain barrier, targets harmful inflammation after most common type of stroke

LiverRight Announces Contactless Remote Patient Monitoring Offering
CINCINNATI, Jan. 6, 2026 /PRNewswire/ — LiverRight, the nation’s only National Virtual Clinic for diagnosing and treating Liver Disease in Adults, announces that its telemedicine appointments in 2026 will offer metrics collection using what’s called Contactless Remote Patient Monitoring (RPM).
TYBR Health reports first clinical use of tissue protector in US
The B3 GEL System maintains biomechanical separation throughout the essential healing window.

Biobeat raises $50M to commercialize cuffless blood pressure monitor
The company will use the money to try to establish its cuffless device as a go-to option for 24-hour blood pressure monitoring in the U.S.

Stereotaxis MAGiC cardiac ablation catheter wins FDA approval
Stereotaxis (NYSE:STXS) has won FDA approval for its robotically navigated MAGiC ablation catheter, offering a minimally invasive option for patients who otherwise might not be eligible for cardiac ablation to treat complex and critical heart rhythm disorders.
Gore wins FDA nod for deep venous stent
W.L. Gore & Associates announced today that the FDA approved its Viabahn Fortegra venous stent, formerly known as Viafort.

Picard Medical to roll out new Syncardia Total Artificial Heart feature
Picard Medical, the company behind the SynCardia Total Artificial Heart (TAH), announced today that it plans to launch a new, FDA-cleared artificial heart accessory.

Schizophrenia and osteoporosis share 195 genetic loci, highlighting unexpected biological bridges between brain and bone
A comprehensive genetic investigation led by Dr. Feng Liu at Tianjin Medical University General Hospital has uncovered striking molecular connections between schizophrenia and bone health, identifying 195 shared genetic loci that may explain why psychiatric patients face elevated fracture risks.
