MedTech News
.................... by Andrew Celentano

Sumbu Unveils World’s First Dual-Vector Consumer Exoskeleton at CES 2026
LAS VEGAS, Jan. 3, 2026 /PRNewswire/ — Today at CES 2026, Sumbu announced its Exo-S3 line of dual-vector exoskeletons designed for the general public: the Exo-S3, Exo-S3 Pro and Exo-S3 Ultra. The series is the world’s first commercially available dual-vector exoskeleton designed to support human movement across real-world terrain. This release establishes a new market for lightweight, AI-powered wearable mobility products.

AI model predicts B cell reactivity to neoantigens for improved cancer vaccines
Professor Jung Kyoon Choi’s research team from the Department of Bio and Brain Engineering, in collaboration with Neogen Logic Co., Ltd., developed the new AI model to predict neoantigens.
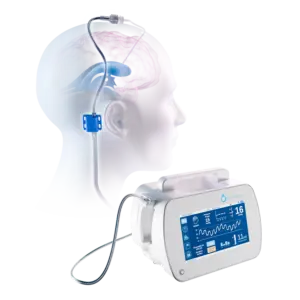
FDA Clears BrainSpace Intellidrop, an automated neuro device that addresses ICU nursing shortages and builds training data for Physical AI
SEATTLE, Jan. 2, 2026 /PRNewswire/ — BrainSpace, a medical technology company building automated solutions for neuro injury, illness and degeneration, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for Intellidrop.

HAI Solutions Receives FDA De Novo Grant for QIKCAP System
HAI Solutions, a leading innovator in ultraviolet (UVC) microbial reduction medical devices, announced that the U.S. Food and Drug Administration (FDA) has granted De Novo classification for its QIKCAP System. This marks the first and only FDA-Granted Ultraviolet light-based microbial reduction device for luer-activated valves, establishing a new Class II medical device category.

StimLabs Announces FDA Clearance of Theracor
Theracor is a sheet device derived from human umbilical cord extracellular matrix (ECM) intended to cover, protect, and provide a moist wound environment

Magnetic pulses to the brain emerge as low-cost lifeline for depression
A major new study has found that transcranial magnetic stimulation (TMS), which applies magnetic energy to the brain, can be a cost-effective treatment option for the NHS in treating moderate and severe forms of depression that have not responded to other treatments.
First breathing ‘lung-on-chip’ developed using genetically identical cells
Researchers at the Francis Crick Institute and AlveoliX have developed the first human lung-on-chip model using stem cells taken from only one person.
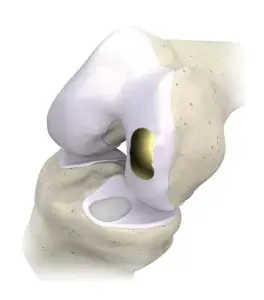
Overture Orthopaedics Announces Milestone $1.0Mn in Sales of its OvertureTi Knee Resurfacing System
OvertureTi Knee Resurfacing System implants are designed specifically as an alternative when it is still too early for arthroplasty
