MedTech News
.................... by Andrew Celentano

Wireless device uses light patterns to deliver information directly to the brain
In a new leap for neurobiology and bioelectronics, Northwestern University scientists have developed a wireless device that uses light to send information directly to the brain—bypassing the body’s natural sensory pathways.

EyeYon Medical Ltd. Receives FDA IDE Approval to Initiate U.S. Clinical Study of EndoArt®, the First Artificial Endothelial Layer for Corneal Edema
NES ZIONA, Israel, Dec. 8, 2025 /PRNewswire/ — EyeYon Medical Ltd., a global leader in ophthalmic innovation, today announced that the U.S. Food and Drug Administration (FDA) has granted Investigational Device Exemption (IDE) approval to initiate a U.S. clinical study of EndoArt®, the world’s first synthetic endothelial layer for the treatment of chronic corneal edema. EndoArt® is currently designated by the FDA as a Breakthrough Device.
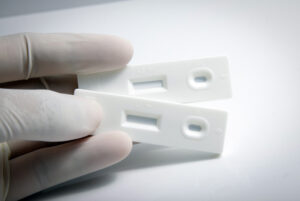
New hepatitis C test paves the way for same-day treatment
15-minute test developed at Northwestern provides results up to 75% faster than current rapid tests

Prognostic tool could help clinicians identify high-risk cancer patients
Using a versatile problem-solving framework, researchers show how early relapse in lymphoma patients influences their chance for survival.
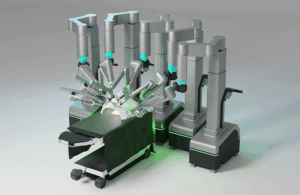
SS Innovations submits Mantra surgical robot to FDA for clearance
SS Innovations (Nasdaq:SSII) announced today that it submitted a 510(k) premarket notification to the FDA for its Mantra surgical robot.

Resmed wins FDA clearance for AI-enabled personalized CPAP therapy device
Resmed (NYSE: RMD)+
announced today that it received FDA clearance for its Smart Comfort personalized therapy offering.
Singapore HSA approves Respiree’s 1Bio AI-Acute toolbox
Healthcare professionals can access the 1Bio AI-Acute toolbox via the company’s 1Bio platform.
AI uncovers how DNA architecture failures trigger blood cancer
Cancer isn’t just about broken genes—it’s about broken architecture. Imagine a city where roads suddenly vanish, cutting off neighborhoods from essential services. That’s what happens inside cells when the 3D structure of DNA collapses.
