MedTech News
.................... by Andrew Celentano
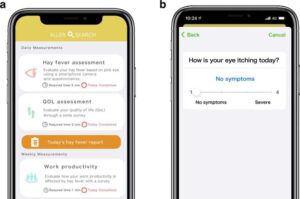
Eye washing may ease hay fever ocular symptoms and improve quality of life
Hay fever, also known as allergic rhinitis, is the condition responsible for seasonal allergies or allergic reactions to other environmental allergens, like dust mites and animal dander. Ultimately, these symptoms disrupt daily activities and affect quality of life for hay fever sufferers.

BD Launches CE-Approved Surgiphor™ Surgical Irrigation System in Europe
BD Surgiphor™ is a sterile, ready-to-use irrigation solution designed to help loosen and remove debris from surgical wounds during procedures

AI detects first imaging biomarker of chronic stress
Using a deep learning AI model, researchers identified the first-of-its-kind biomarker of chronic stress detectable through routine imaging, according to research presented at the annual meeting of the Radiological Society of North America (RSNA).

Virtual retina could help unlock new vision loss treatments
New computer modeling could help scientists better understand how the retina regenerates, opening the door to new treatments for vision loss, according to a study from the University of Surrey.

3D map sheds light on why tendons are prone to injury
Scientists at the University of Portsmouth have created the first detailed 3D map of how a crucial piece of connective tissue in our bodies responds to the stresses of movement and exercise. This tissue, called calcified fibrocartilage (CFC), acts like a biological shock absorber where tendons attach to bone.

Philips launches next-generation web-based diagnostic viewer for fast, secure imaging data access
New viewer offers full radiology capabilities, interfaces with AI and interactive reporting module to streamline workflows

InventHelp Inventor Develops Improved Breast Pump (LBT-9001)
PITTSBURGH , Nov. 25, 2025 /PRNewswire/ — “I thought there should be a way for my wife to get a restful night of sleep while still pumping to maintain the milk supply for feeding our baby,” said an inventor, from Campbellsburg, Ky., “so I invented the AUTO – PUMPING BRA WITH TIIMER FUNCTION. My design helps exhausted moms by eliminating the need to choose between pumping and sleeping.”
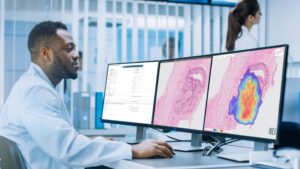
Nucs AI Expands AI-Driven Prostate Cancer Platform With Launch of TrackPSMA
NEW YORK, Nov. 25, 2025 /PRNewswire/ — Nucs AI, the leader in AI-based radiology solutions, today announced the launch of TrackPSMA, a breakthrough tool that gives physicians a faster, quantitative way to evaluate treatment response in prostate cancer.
