MedTech News
.................... by Andrew Celentano
French scientists probe mRNA’s potential to fight cancer
Inside a lab in the French city of Orleans, scientists are testing out the limits of molecules in our body called messenger RNA—best known for being used in COVID-19 vaccines—in the hopes of finding a breakthrough treatment for a particularly deadly cancer.
Scientists use stem cells to model rare genetic blindness in children
Researchers at the Eye Genetics Research Unit at Children’s Medical Research Institute (CMRI) are the first in the world to use stem cells to study one of the genetic causes of Leber Congenital Amaurosis (LCA)—a rare condition that causes severe vision loss in babies and young children.
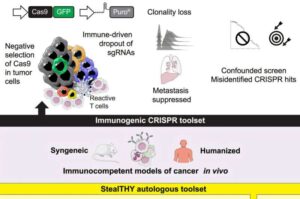
Gene scissors in camouflage mode help in the search for cancer therapies
The immune system plays a crucial role in fighting tumors and metastases. Consequently, it is decisive to conduct cancer research in mouse models with an immune system that is as natural as possible—which is easier said than done.
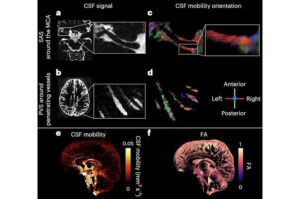
Cerebrospinal fluid motion in the brain captured in remarkable detail
Cerebrospinal fluid (CSF) is a clear and watery liquid that flows in and around the brain and spinal cord. Its functions include protecting parts of the nervous system, delivering nutrients and removing metabolic waste.
Fujifilm Sonosite launches AI-powered ultrasound tech for peripheral intervention
Fujifilm Sonosite announced today that it launched PIV Assist, a new, AI-powered feature for planning peripheral intravenous (PIV) access procedures.
Dexcom wins FDA nod for smart CGM-integrated basal dosing for type 2 diabetes
Dexcom (NSDQ:DXCM) announced today that the FDA cleared its Smart Basal CGM-integrated basal insulin dosing optimizer.

Bandage-like device brings texture to touchscreens
Soft, stretchable VoxeLite wraps around a fingertip to let users feel the digital world
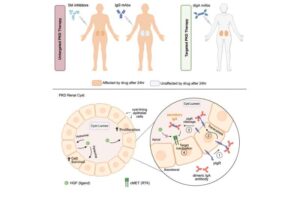
A ‘magic bullet’ for polycystic kidney disease in the making
Polycystic kidney disease (PKD) is a debilitating hereditary condition in which fluid-filled sacs form and proliferate in the kidneys. Over time, the painful, growing cysts rob the organs of their function, often leading to dialysis in advanced cases. There is currently no cure.
