MedTech News
.................... by Andrew Celentano
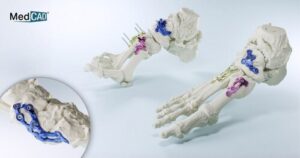
MedCAD’s AccuStride™ Fixation Plates Receive 510(k) FDA Clearance to Complete Foot and Ankle System Offering
DALLAS, Nov. 6, 2025 /PRNewswire/ — Dallas-based MedCAD has been awarded 510(k) FDA clearance for its AccuStride fixation plates, part of a patient-specific solution available to surgeons as a complete foot and ankle (F&A) system. The company received 510(k) FDA clearance for its foot and ankle guides and planning system in March 2025. The AccuStride implant and surgical guide system coupled with the company’s proprietary planning software will enable foot and ankle surgeons to correct multiple related pathologies in a single procedure.

Nitinotes Receives CE Mark Approval of EndoZip™, the First Fully Automated Suturing System for Endoscopic Sleeve Gastroplasty
CAESAREA, Israel, Nov. 6, 2025 /PRNewswire/ — Nitinotes Ltd., a medical device company transforming the treatment of obesity, today announced it has received CE Mark approval for the EndoZip™ System, the first fully automated suturing platform for endoscopic sleeve gastroplasty (ESG). The milestone clearance enables Nitinotes to begin commercialization across the European Union and other CE Mark-accepting markets.

Microfluidic sensors enable real-time sweat analysis
Eccrine sweat is a water-like fluid secreted by eccrine sweat glands that comprises various kinds of biochemical components such as electrolytes, metabolites, organic molecules, and drugs.

Review finds no link between acetaminophen use in pregnancy and neurodevelopmental disorders
A rigorous systematic review of the present state of knowledge on the use of acetaminophen during pregnancy and the risk of specific neurodevelopmental disorders (NDDs), such as autism and ADHD, offers reassurance that acetaminophen does not increase the risk of NDDs.
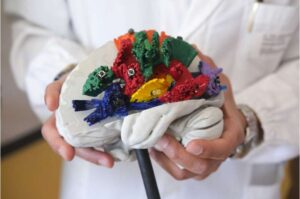
3D atlas merges brain dissection and imaging for detailed white matter mapping
BraDiPho (Brain Dissection Photogrammetry) is an innovative tool for the study of white matter connections in the human brain. The realistic map was developed by a group of researchers from the University of Trento, the Provincial Healthcare Service of Trento, Fondazione Bruno Kessler and the Universities of Bordeaux and Sherbrooke.
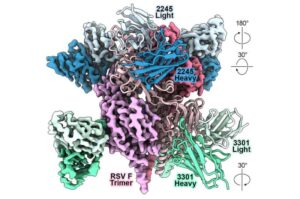
AI can speed antibody design to thwart novel viruses
Artificial intelligence (AI) and “protein language” models can speed the design of monoclonal antibodies that prevent or reduce the severity of potentially life-threatening viral infections, according to a multi-institutional study led by researchers at Vanderbilt University Medical Center.
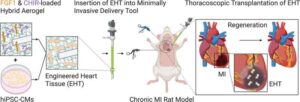
Researchers identify a new stem cell patch to gently heal damaged hearts
Mayo Clinic researchers have developed a pioneering method to mend damaged hearts without open-heart surgery, an advance that could one day transform the treatment of heart failure.

Simple-to-use tech could help former soldiers readjust to civilian life
A newly discharged American military veteran struggles emotionally to quiet memories from the battlefield. He smokes cannabis, increasingly, to fall asleep at night and to get through the day.
