MedTech News
.................... by Andrew Celentano

Scientists create artificial retina phantom to standardize eye disease diagnosis equipment
The Korea Research Institute of Standards and Science (KRISS) has developed a retina-mimicking eye phantom that faithfully replicates the structural layers and microvascular network of the human retina. This innovation provides a new reference for objectively evaluating and calibrating ophthalmic imaging devices, paving the way for more accurate and reliable diagnosis of retinal diseases.

CRISPR screen identifies new regulator of androgen receptor in prostate cancer
A poorly characterized protein, historically thought to be a chaperon or enzyme, may actually be a key player in prostate cancer. In a systematic CRISPR screen, scientists from Arc Institute, UCSF, and the Fred Hutchinson Cancer Center have identified PTGES3, known as the third prostaglandin E synthase protein, as an unexpected regulator of the androgen receptor.
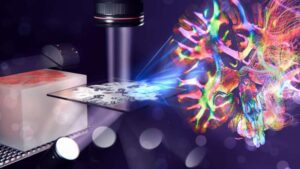
Imaging technique maps the brain’s nerve fiber labyrinth with micrometer precision
In order to understand brain diseases, neuroscientists try to untangle the intricate nerve fiber labyrinth of our brain. Before analyzing brain tissue under a microscope, it is often soaked in paraffin wax to achieve high-quality sections. However, accurately mapping the densely packed nerves inside wax-treated brain slices was so far not possible.

Caricature-inspired brain mapping method sharpens forecasts of cognitive and emotional traits
Caricature artists exaggerate distinctive features of an individual, deepening a cleft chin or multiplying freckles. Yale researchers have now applied a similar approach to maps of neural connections, emphasizing individual differences to see if they yield useful information.
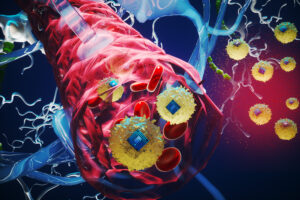
New therapeutic brain implants could defy the need for surgery
MIT researchers created microscopic wireless electronic devices that travel through blood and implant in target brain regions, where they provide electrical stimulation.

MIT researchers invent new human brain model to enable disease research, drug discovery
Cultured from induced pluripotent stem cells, “miBrains” integrate all major brain cell types and model brain structures, cellular interactions, activity, and pathological features.

How Femasys Is Using Innovation to Rethink Permanent Birth Control
Designed as a safe, accessible, and office-based alternative to surgical sterilization, FemBloc represents a paradigm shift in permanent contraception
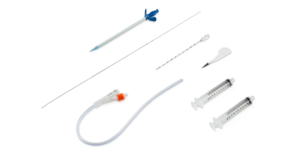
Mediplus expands S-Cath range with launch of S-Cath System Long
Mediplus has expanded its award-winning S-Cath System range for suprapubic catheterisation with the launch of S-Cath System Long, featuring longer components and enhanced features to give clinicians greater choice, flexibility, and improved usability.
