MedTech News
.................... by Andrew Celentano

Regenity Biosciences Marks 63rd FDA 510(k) Clearance with Expansion of Neurosurgical Portfolio
PARAMUS, N.J., Nov. 25, 2025 /PRNewswire/ — Regenity Biosciences, a global leader in regenerative medicine and Linden Capital Partners portfolio company, today announced its 63rd FDA 510(k) clearance for DuraMatrix® Repair – Collagen Dura Regeneration Membrane, a resorbable membrane composed of highly purified Type I and Type III bovine collagen for the repair of dura mater defects during neurosurgery.
Nasal nanomedicine delivers immune-boosting therapy to fight brain tumors
Researchers at Washington University School of Medicine in St. Louis, along with collaborators at Northwestern University, have developed a noninvasive approach to treat one of the most aggressive and deadly brain cancers.

CMS approves coverage for Recor Medical renal denervation study
Recor Medical said today that it secured Medicare approval, granting coverage for the company’s RADIANCE CED study.

Overture Life gains CE mark for automated IVF system
Overture’s DaVitri system aims to make egg thawing and freezing processes during IVF more consistent to improve successful embryo formation outcomes.
QuantalX gains de novo classification for neuro-imaging system
QuantalX’s Delphi-MD is applicable for the diagnosis, monitoring, and treatment evaluation of a range of neurological conditions.

New implant captures gut-brain signals in awake, moving animals
Scientists have been able to measure the electrical signals in the “second brain in our guts” for the first-ever time, giving renewed understanding to its interconnection with the brain.
Paradromics gets FDA green light for BCI (Brain Computer Interface) study
Paradromics announced today that it received FDA investigational device exemption (IDE) to begin a study of its brain-computer interface (BCI).
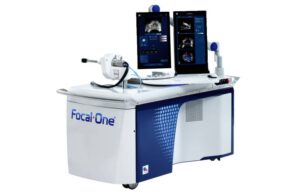
EDAP wins FDA nod for robotic high-intensity focused ultrasound system workflows
EDAP TMS SA (Nasdaq:EDAP) announced today that the FDA granted 510(k) clearance for new workflows for its ultrasound technology.
