MedTech News
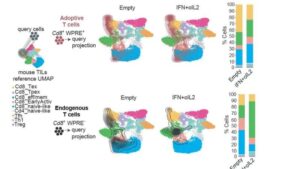
Engineered cell cross-talk unlocks CAR-T potential against glioblastoma
A team of researchers from the San Raffaele-Telethon Institute for Gene Therapy (SR-TIGET, Milan), led by Nadia Coltella and Luigi Naldini, has unveiled a powerful strategy to rejuvenate the effectiveness of chimeric antigen receptor (CAR) T cell therapy against glioblastoma, one of the most lethal and treatment-resistant brain tumors.

AI predicts patients likely to die of sudden cardiac arrest
A new AI model is much better than doctors at identifying patients likely to experience cardiac arrest. The linchpin is the system’s ability to analyze long-underused heart imaging, alongside a full spectrum of medical records, to reveal previously hidden information about a patient’s heart health.
Intuitive wins CE mark for da Vinci 5
Intuitive Surgical (Nasdaq: ISRG)+
announced today that it received CE mark for its da Vinci 5 surgical robotic system.

Fasikl wins FDA nod for tremor-treating wearable wristband
Fasikl announced that it received FDA 510(k) clearance for its first-of-its-kind NeuroAI wristband for essential tremor.
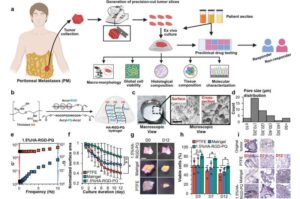
Bioengineered hydrogel mimics tumor environment to preserve live tissues for longer
A new hydrogel-based platform to preserve live patient-derived tumor tissues in the lab could pave the way for faster, more accurate testing of cancer treatments for patients with peritoneal metastases, a hard-to-treat and often deadly form of abdominal and pelvic cancers.

Researchers take major step toward cuff-free blood pressure monitoring
Researchers have shown, for the first time, that speckle contrast optical spectroscopy (SCOS) can be used for cuffless blood pressure monitoring. The new technology could improve early detection and management of hypertension.

Moon Surgical Receives FDA Clearance for Maestro™ Connectivity and Predetermined Change Control Plan for AI-Powered ScoPilot®
PARIS and SAN FRANCISCO, July 2, 2025 /PRNewswire/ — Moon Surgical, today announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for two major advancements to its Maestro System: connectivity of the platform to power the Maestro Insights product and a Predetermined Change Control Plan (PCCP) to evolve its AI-powered ScoPilot product.

Lifecare takes next steps in CGM implant development
Lifecare said on social media that it completed a significant milestone in the development of its continuous glucose monitor (CGM) implant.
