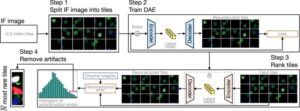
Automated algorithm can detect cancer in blood samples in as little as 10 minutes
When cancer spreads, tiny amounts of cells can break away from tumors and circulate in the bloodstream. A liquid biopsy is a means to detect the presence of cancer by detecting these cancer cells floating in blood samples. However, current state-of-the-art methods have necessitated trained specialists to comb through and review images of thousands of cells out of potentially millions of cells on a slide over a period of many hours.