
AI model can accelerate antibody drug production
Researchers detail a machine learning model that dramatically accelerates the manufacturing timeline of monoclonal antibodies.

Researchers detail a machine learning model that dramatically accelerates the manufacturing timeline of monoclonal antibodies.
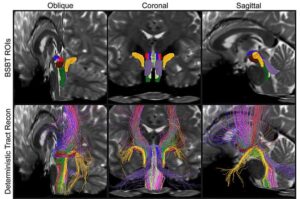
Research reveals distinct patterns of structural changes in patients with Parkinson’s disease, multiple sclerosis, and traumatic brain injury.

SEOUL, South Korea, Feb. 6, 2026 /PRNewswire/ — Neurophet (Co-CEOs Jake Junkil Been and Donghyeon Kim), an artificial intelligence (AI) solution company for brain disorders diagnosis and treatment, announced today that its software solution, Neurophet AQUA AD Plus, a comprehensive neuroimaging analysis solution for clinical evaluation related to Alzheimer’s disease, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).

Dexcom (NSDQ:DXCM) announced today that it plans to launch an advanced AI-enabled enhancement to its Stelo over-the-counter CGM.
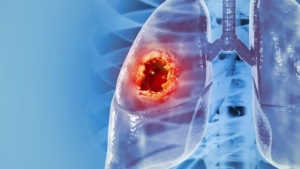
RevealDX’s software analyses CT scans and assigns lung nodules with a Malignancy Similarity Index score to aid in lung cancer diagnosis.

As humans age beyond early adulthood, their physical and mental functions tend to slowly worsen over time. One of the most common sources of severe mental decline in older adults are neurodegenerative diseases, conditions characterized by the progressive loss of neurons in the brain or peripheral nervous system.
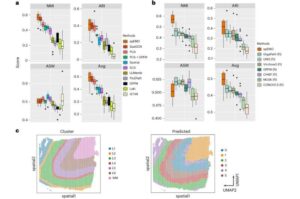
A new study from Yale University researchers shows how artificial intelligence can bring image, gene and protein data together, offering a clearer picture of what is happening inside the body and how diseases develop.

Cancer patients who suffer a heart attack face a dangerous mix of risks, which makes their clinical treatment particularly challenging. As a result, patients with cancer have been systematically excluded from many clinical trials and available risk scores. Until now, doctors had no standard tool to guide treatment in this vulnerable group.

Veritas.AI is designed to advance the functionality of Spectrum Dynamics’ VERITON-CT scanner for nuclear imaging.

SARASOTA, Fla., Jan. 29, 2026 /PRNewswire/ — Spectrum Dynamics Medical, a global leader in digital nuclear medicine imaging solutions, today announced that it has received FDA 510(k) clearance for Veritas.AI™ Noise Reduction, its advanced artificial intelligence platform designed to significantly enhance image quality, diagnostic confidence, and operational efficiency on the VERITON-CT® digital SPECT/CT system.