
CeriBell gets FDA nod for neonate seizure detection algorithm
CeriBell (Nasdaq:CBLL) announced today that it received FDA 510(k) clearance for its next-generation Clarity algorithm.

CeriBell (Nasdaq:CBLL) announced today that it received FDA 510(k) clearance for its next-generation Clarity algorithm.

PARAMUS, N.J., Nov. 25, 2025 /PRNewswire/ — Regenity Biosciences, a global leader in regenerative medicine and Linden Capital Partners portfolio company, today announced its 63rd FDA 510(k) clearance for DuraMatrix® Repair – Collagen Dura Regeneration Membrane, a resorbable membrane composed of highly purified Type I and Type III bovine collagen for the repair of dura mater defects during neurosurgery.

Recor Medical said today that it secured Medicare approval, granting coverage for the company’s RADIANCE CED study.

Overture’s DaVitri system aims to make egg thawing and freezing processes during IVF more consistent to improve successful embryo formation outcomes.
QuantalX’s Delphi-MD is applicable for the diagnosis, monitoring, and treatment evaluation of a range of neurological conditions.
Paradromics announced today that it received FDA investigational device exemption (IDE) to begin a study of its brain-computer interface (BCI).
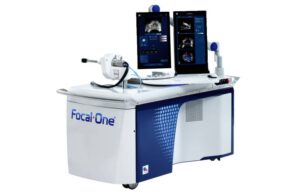
EDAP TMS SA (Nasdaq:EDAP) announced today that the FDA granted 510(k) clearance for new workflows for its ultrasound technology.
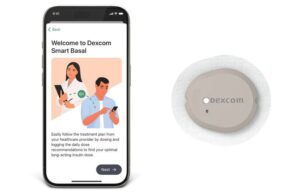
Dexcom (NSDQ:DXCM) announced today that the FDA cleared its Smart Basal CGM-integrated basal insulin dosing optimizer.

ELLSWORTH, Maine, Nov. 19, 2025 /PRNewswire/ — Rejuva Fresh®, a global leader in non-invasive aesthetic and wellness solutions, proudly announces expanded CE certification and design upgrades for its revolutionary EMSTRONG® platform, reinforcing its leadership in pelvic health and intimate wellness.

Having been commercially available for less than 12 months, the system is now offered in over 60 US clinics.