GE HealthCare wins FDA nod for next-gen Signa MRI tech
GE HealthCare (Nasdaq: GEHC)+ announced today that it received FDA clearance for a trio of new MRI offerings.

GE HealthCare (Nasdaq: GEHC)+ announced today that it received FDA clearance for a trio of new MRI offerings.

LOUISVILLE, Ky., Feb. 19, 2026 /PRNewswire/ — Breath Diagnostics, Inc., a leader in breath-based molecular diagnostics powered by patented microreactor capture technology, is pleased to announce that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to its OneBreath™ platform.

DAYTON, Ohio, Feb. 19, 2026 /PRNewswire/ — Innovative Sterilization Technologies (IST) announced today that the ONE TRAY® sterilization container has received a second clearance from the U.S. Food and Drug Administration (FDA), significantly expanding its use across hospital and surgical facility workflows.

CHICAGO, Feb. 19, 2026 /PRNewswire/ — Sibel Health, a leader in medical-grade wearable sensor technology, today announced that the U.S. Food and Drug Administration (FDA) has accepted the company’s recent Letter of Intent (LOI) into the Clinical Outcome Assessment (COA) Qualification Program under the Drug Development Tool (DDT) framework. The acceptance marks a significant milestone in advancing objective cough frequency measurement for adult patients with chronic refractory cough (CRC) using a novel Cough Monitoring sensor, the Aria sensor.
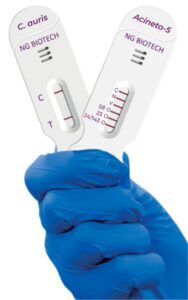
NG Biotech manufactured the assays in France, with Hardy Diagnostics serving as the exclusive distributor in the US.
Medtronic (NYSE: MDT)+ announced today that it received FDA premarket approval (PMA) for the use of its Infuse bone graft in TLIF procedures.
The system is intended for use in urgent care clinics, primary care settings, physician office laboratories, and pharmacies.

WESTFIELD, Ind., Feb. 17, 2026 /PRNewswire/ — Today, Portal Diabetes, Inc. (“Portal”) announced its receipt of the Breakthrough Device Designation by the Food and Drug Administration (FDA) for its implantable insulin pump system called “Portal Pump,” and the start of a Phase 1 study on its proprietary temperature-stable insulin (“Portal Insulin”).
WHITE PLAINS, N.Y., Feb. 17, 2026 /PRNewswire/ — Retia Medical today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for Argos Infinity™, the company’s cardiovascular intelligence software platform designed for high-risk surgical and critical care environments across health systems.

MURRIETA, Calif., Feb. 17, 2026 /PRNewswire/ — Copan Group announced today that PhenoMATRIX®, its automated image assessment software used with WASPLab® full laboratory automation, received FDA 510(k) clearance as a Class II device in the United States.