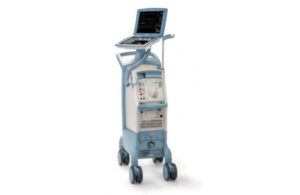
Getinge earns reinstated CE mark for intra-aortic balloon pump
Getinge announced today that European regulatory authorities reinstated the CE mark for its Cardiosave intra-aortic balloon pump.

Getinge announced today that European regulatory authorities reinstated the CE mark for its Cardiosave intra-aortic balloon pump.
Carlsmed (Nasdaq:CARL) today announced new technology add-on payment (NTAP) reimbursement for its spine implant technology.
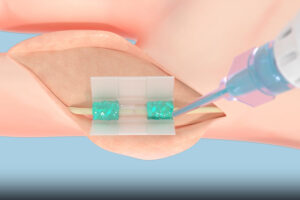
MIT spinout Tissium recently secured FDA marketing authorization of a biopolymer platform for nerve repair.
EvoEndo announced today that it received expanded FDA clearance for its Model LE gastroscope, covering patients of all ages.
SetPoint Medical announced today that the FDA approved its SetPoint neuroimmune modulation system for treating rheumatoid arthritis (RA).
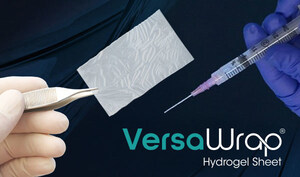
AUSTIN, Texas, July 30, 2025 /PRNewswire/ — Alafair Biosciences, a medical device company redefining soft-tissue protection in orthopedic surgery, announced today that it has received two 510(k) clearances from the FDA for VersaCoat™ Tendon Protector and VersaCoat™ Nerve Protector, marketed as a single product, VersaCoat™ Flowable Hydrogel.

Bioventus (Nasdaq:BVS) announced today that it received FDA 510(k) clearance for both its TalisMann and StimTrial offerings.

FRANKLIN LAKES, N.J., July 30, 2025 /PRNewswire/ — BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the BD Veritor™ System for SARS-CoV-2, a digital test designed to detect COVID-19 antigens in symptomatic individuals in about 15 minutes at doctors’ offices, urgent care centers, retail clinics and other convenient points of care.

VersaCoat, a syringe-deliverable soft-tissue protector, brings Alafair’s proven hyaluronic acid and alginate formula to complex surgical sites—expanding its impact in orthopedic, trauma, and sports medicine procedures.

BURLINGTON, N.C., July 29, 2025 /PRNewswire/ — Labcorp (NYSE: LH), a global leader of innovative and comprehensive laboratory services, announced today PGDx elio™ tissue complete has been CE-marked under the European Union’s (EU) new In Vitro Diagnostic Regulation (IVDR).