Leo Cancer Care Receives FDA 510(k) Clearance for Marie® – A Revolutionary Upright Radiotherapy Platform
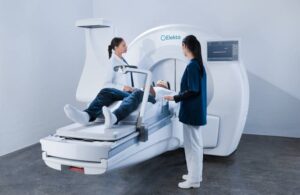
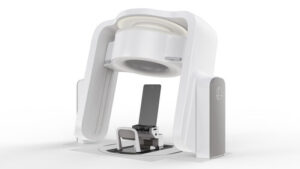
MIDDLETON, Wis., July 29, 2025 /PRNewswire/ — Leo Cancer Care, a leader in upright radiotherapy solutions, today announces that its flagship product, Marie®, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).