
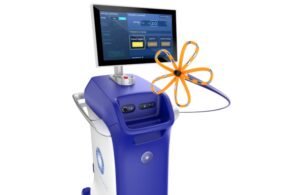
Mendaera wins FDA clearance for robotic needle placement
Mendaera has received FDA 510(k) clearance for its Focalist handheld robotic system, which enhances the precision of ultrasound-guided needle placement across multiple specialties.

Mendaera has received FDA 510(k) clearance for its Focalist handheld robotic system, which enhances the precision of ultrasound-guided needle placement across multiple specialties.

FREMONT, Calif., July 8, 2025 /PRNewswire/ — Q’Apel Medical (Q’Apel), a private medical device company focused on revolutionizing neurovascular interventions, today announced it has received U.S. Food and Drug Administration (FDA) clearance for its Zebra Neurovascular Access System. Available in 6F and 7F sizes, Zebra is indicated for the introduction of interventional devices into the peripheral and neurovasculature.
Boston Scientific (NYSE: BSX)+
announced today that the FDA approved an expansion to the label of its Farapulse pulsed field ablation (PFA) system.

FDA clears assay as companion diagnostic for ZEGFROVY™ and broad tumor profiling, advancing precision oncology diagnostics.

Dual AI-Powered Imaging Software Set to Enhance Diagnostic Confidence and Workflow Efficiency in MRI
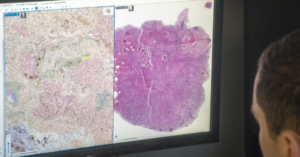
PathAI, a global leader in artificial intelligence (AI) and digital pathology solutions, has announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for AISight Dx—its digital pathology image management system—for use in primary diagnosis in clinical settings.
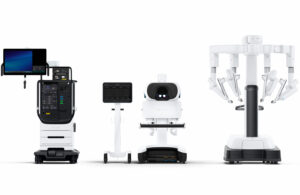
Intuitive Surgical (Nasdaq: ISRG)+
announced today that it received CE mark for its da Vinci 5 surgical robotic system.

Fasikl announced that it received FDA 510(k) clearance for its first-of-its-kind NeuroAI wristband for essential tremor.

PARIS and SAN FRANCISCO, July 2, 2025 /PRNewswire/ — Moon Surgical, today announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for two major advancements to its Maestro System: connectivity of the platform to power the Maestro Insights product and a Predetermined Change Control Plan (PCCP) to evolve its AI-powered ScoPilot product.
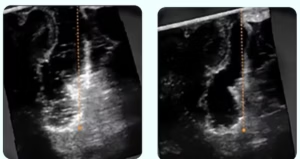
OSLO, Norway, July 1, 2025 /PRNewswire/ — SonoClear AS today announced that the Center for Devices and Radiological Health (CDRH) of the U.S. Food and Drug Administration (FDA) has designated the SonoClear® System as a Breakthrough Device for use in intracranial ultrasound procedures.