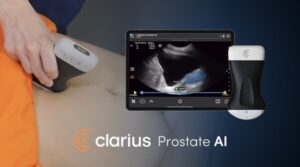
FDA Clears New Clarius Prostate AI To Help Physicians Assess and Diagnose Common Urologic Conditions in Minutes
Clarius is the first to offer an FDA-cleared AI-powered prostate measurement tool on a handheld ultrasound scanner—empowering primary care and urology physicians to assess patients on the spot without waiting for specialist referrals