Terumo Neuro Receives FDA Approval for Carotid Stent System
First Dual-Layer Micromesh Carotid Stent Approved for Use in the U.S.

First Dual-Layer Micromesh Carotid Stent Approved for Use in the U.S.

Dexcom (Nasdaq: DXCM) today announced a significant regulatory milestone for its continuous glucose monitoring (CGM) technology.

Intuitive (Nasdaq: ISRG) announced today that it received FDA clearance for its fully wristed SP SureForm 45 stapler.

Zeiss Medical Technology announced today that it received FDA 510(k) clearance for the Intrabeam 700 platform.

New Approval Expands Use of Porous Biologic Scaffold for Intervertebral Disc Space Fusion Procedures

HighLife announced that it received FDA breakthrough device designation for its trans-septal mitral valve replacement (TMVR) solution.
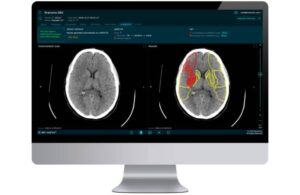
Brainomix announced today that it received FDA clearance for a new feature within its 360 Stroke solution for AI-powered stroke imaging.

Proprio announced today that it received its second FDA 510(k) clearance for its Paradigm AI guidance platform for surgery.

CardioVia announced today that it received FDA clearance for its minimally invasive ViaOne epicardial access system.

Advanced Dosimetry Software Expands Capabilities for Personalized Radiopharmaceutical Therapies