
FDA clears new stemless anatomical total shoulder implant from Smith+Nephew
Smith+Nephew (NYSE: SNN)+
announced that it received FDA clearance for its stemless anatomic total shoulder for the Aetos system.

Smith+Nephew (NYSE: SNN)+
announced that it received FDA clearance for its stemless anatomic total shoulder for the Aetos system.

DÜSSELDORF, Germany, Dec. 16, 2024 /PRNewswire/ — Gerresheimer, an innovative system and solution provider and a global partner for the pharma, biotech and cosmetics industries, announces that the US Food and Drug Administration (FDA) granted SQ Innovation Tentative Approval for Lasix ONYU for the home treatment of fluid overload in congestive heart failure.

The Osseofit devices are intended to match patients’ shoulder bone anatomy and preserve healthy bone in total shoulder replacement procedures.

HeartBeam (Nasdaq:BEAT) announced today that it received FDA 510(k) clearance for its comprehensive arrhythmia assessment system.

Withings Health Solutions announced today that it received FDA clearance for BPM Pro 2, a first-of-its-kind cellular blood pressure monitor.

ALBUQUERQUE, N.M., Dec. 16, 2024 /PRNewswire/ — Gastro Concepts, a team committed to advancing safety and efficiency in gastroenterology, today announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its groundbreaking Air Assist™ device.

A pivotal study of the robotic platform in colorectal surgery will begin at five hospitals in early 2025.

FemPulse announced today that it received FDA investigational device exemption (IDE) approval for its overactive bladder (OAB) therapy.

MONTVALE, N.J., Dec. 10, 2024 /PRNewswire/ — PENTAX Medical, a division of HOYA Group, has obtained US FDA 510(k) clearance for new models of the PENTAX Medical i20c Video Endoscope Series—PENTAX Medical Video Colonoscope EC34-i20cL, PENTAX Medical Video Upper GI Scope EG27‑i20c and Right/Left Wheel Extender OE-B17.
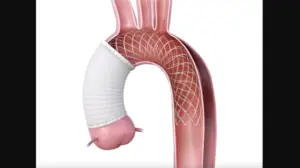
Artivion (NYSE:AORT) this week announced it received FDA humanitarian device exemption (HDE) for its AMDS hybrid prosthesis.