Ultromics obtains FDA clearance for Cardiac Amyloidosis screening device
EchoGo Amyloidosis is an AI-based software-only medical device, which aims to improve early detection of Cardiac Amyloidosis, an underdiagnosed cause of heart failure.

EchoGo Amyloidosis is an AI-based software-only medical device, which aims to improve early detection of Cardiac Amyloidosis, an underdiagnosed cause of heart failure.
The device has sold well in Europe, where the company said it has a 60% market share, but will be the first product of its type available in the U.S.
SEOUL, South Korea, Nov. 25, 2024 /PRNewswire/ — Neurophet, an artificial intelligence (AI) solution company for brain disease, announced on the 25th that its brain MRI analysis software “Neurophet AQUA”, has obtained 510(k) clearance from U.S. Food and Drug Administration (FDA) for its newly integrated multiple sclerosis (MS) analysis functionality.
MILPITAS, Calif., Nov. 25, 2024 /PRNewswire/ — LifeSignals, Inc. today announced that the UbiqVue 2A Multiparameter System has received FDA Class II 510(k) clearance, marking a significant milestone in continuous wireless patient monitoring for population health management.

President-elect Donald Trump has nominated Johns Hopkins University surgeon Dr. Martin Makary to be the next commissioner of the FDA, according to media reports.
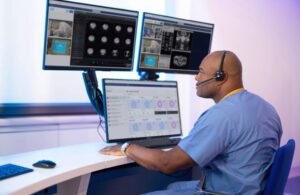
Philips (NYSE:PHG) announced today that it received FDA 510(k) clearance for a number of remote imaging features.

The clearance paves the way for the launch of Medtronic’s Smart MDI system, which combines its InPen and Simplera continuous glucose monitor for people who take multiple daily insulin injections.

Align Technology (Nasdaq:ALGN) announced today that it received CE mark for its Invisalign palatal expander system.

The RESPOND ventilator expands access to care with a 5-year warranty and minimal maintenance needs, enhancing long-term affordability.
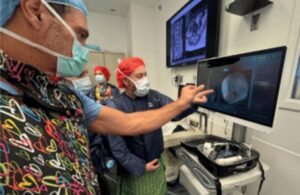
Augmedics announced today that the FDA cleared the CT-to-fluoroscopy (CT-Fluoro) registration method for its Xvision Spine System.