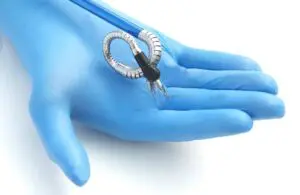
Momentis Surgical wins FDA clearance for its surgical robot
Momentis Surgical (formerly Memic) announced today that it received FDA 510(k) clearance for its Anovo robotic surgical platform.

Momentis Surgical (formerly Memic) announced today that it received FDA 510(k) clearance for its Anovo robotic surgical platform.
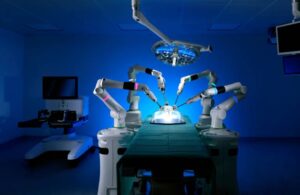
CMR Surgical announced today that it received FDA de novo clearance to market its Versius surgical robot platform.

Medtronic (NYSE: MDT)+ announced today that the FDA approved an early feasibility study to evaluate its Affera system for treating VT.

Iota Biosciences, a subsidiary of Astellas Pharma, announced that it received FDA investigational device exemption (IDE) for its bladder implant.

AccurKardia announced that it received FDA breakthrough device designation for its aortic valve stenosis (AVS) ECG-based AI screening software.
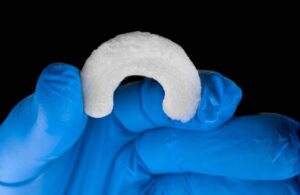
Regenity Biosciences announced that it received FDA 510(k) clearance for its RejuvaKnee implant for soft tissue injuries of the meniscus.
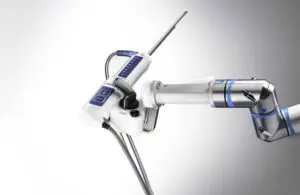
Procept BioRobotics (Nasdaq:PRCT) announced today that the FDA approved an investigational device exemption (IDE) trial for its Aquablation therapy.

CHICAGO, Oct. 3, 2024 /PRNewswire/ — Amphix Bio, a company developing a new class of regenerative medicine therapies, announced today it has received a Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for a drug-device combination product for bone regeneration. The designation covers the use of the therapeutic device to treat degenerative disc disease with transforaminal lumbar interbody fusion (TLIF) procedures.
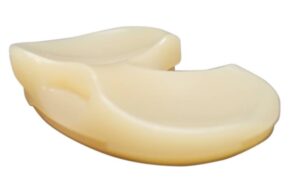
Corin announced that it received FDA 510(k) clearance for its Unity Knee medial constrained (MC) tibial insert.
Pi-Cardia designed the Shortcut device to mitigate the risk of coronary artery obstruction by splitting aortic valve leaflets before valve placement.