Surgical Planning Associates wins FDA nod for mixed-reality hip arthroplasty system
Surgical Planning Associates announced today that it received FDA 510(k) clearance for its HipInsight 2.0 mixed-reality guidance system.

Surgical Planning Associates announced today that it received FDA 510(k) clearance for its HipInsight 2.0 mixed-reality guidance system.
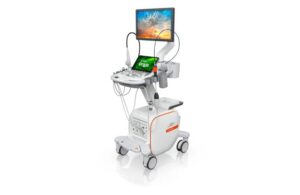
Siemens Healthineers announced today that it received FDA clearance for its Acuson Origin ultrasound system and AcuNav Lumos 4D ICE catheter.
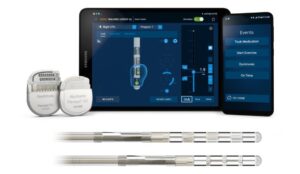
Medtronic says the approval provides more options to meet patients’ personalised needs, including those with Parkinson’s disease.
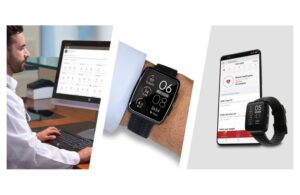
Masimo (Nasdaq: MASI)+
announced today that the FDA granted 510(k) clearance for its W1 medical watch for connectivity purposes
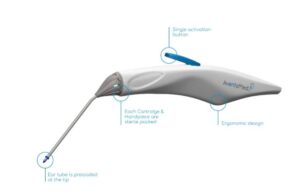
AventaMed, a Karl Storz company, announced that it received FDA 510(k) clearance for its Solo+ ear tube placement system.

Medtronic (NYSE: MDT)+ got another boost for its CGM portfolio, adding to the collaboration with Abbott announced today.
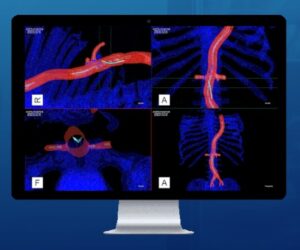
CLEVELAND, Aug. 6, 2024 /PRNewswire/ — Centerline Biomedical, Inc. (“Centerline”), an innovation leader in cardiovascular navigation and visualization systems, announced today that the IOPS Viewpoint Catheter has received US Food and Drug Administration (FDA) 510(k) clearance. The Viewpoint Catheter is the most recent addition to the company’s patented IOPS (Intra-Operative Positioning System) portfolio.

Diality announced today that it received FDA 510(k) clearance for its Moda-flx smart, flexible hemodialysis system.

Pentax Medical announced today that the FDA cleared its DEC duodenoscope compatibility with the Sterrad 100NX sterilizer.
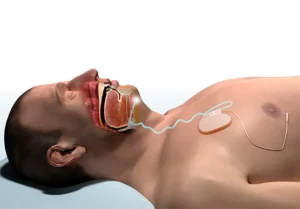
Inspire Medical Systems (NYSE: INSP)+ announced today that it received FDA approval for its Inspire V therapy system.