MiRus wins FDA breakthrough nod for spine implant
MiRus announced that it received FDA breakthrough device designation for its Europa posterior cervical system for the spine.

MiRus announced that it received FDA breakthrough device designation for its Europa posterior cervical system for the spine.

Stryker (NYSE: SYK)+
announced today that it received FDA 510(k) clearance for its Q Guidance System with Spine Guidance 5 software featuring Copilot.
Siemens Healthineers‘ Varian announced that it received FDA 510(k) clearance for its IntelliBlate microwave ablation system.

Extension to Indications for Use follows submission of results from a human factors study evaluating the safe and effective use of the device in the home care setting.
CorVascular announced today that the FDA granted clearance for its VasoGuard V-Series portfolio of devices.
Asensus Surgical (NYSE:ASXC) announced today that it received FDA 510(k) clearance for an expanded surgical robot indication.
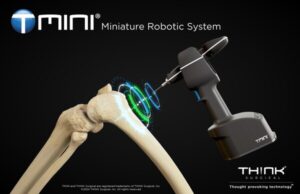
FREMONT, Calif., July 23, 2024 /PRNewswire/ — THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, today announced that its TMINI® Miniature Robotic System (TMINI 1.1) has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).

eCential Robotics announced today that it received FDA 510(k) clearance for its spine navigation and robotic-assistance device.
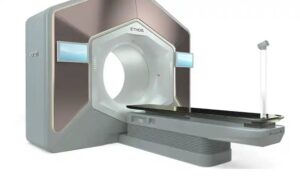
Siemens Healthineers‘ Varian announced today that it received FDA 510(k) clearance for a new functionality for its Ethos therapy system.

The South Korean company’s Epysqli is now FDA-approved for paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome in the U.S., having grabbed the lead in the Soliris biosimilar market in Europe.