KORU Medical seeks FDA clearance for infusion system
KORU Medical has submitted a 510(k) premarket notification to the US Food and Drug Administration (FDA) seeking approval for its FreedomEDGE infusion system.

KORU Medical has submitted a 510(k) premarket notification to the US Food and Drug Administration (FDA) seeking approval for its FreedomEDGE infusion system.

Scientists at Johns Hopkins Medicine say results of a new study are advancing efforts to exploit a new target for Alzheimer’s disease: a protein that manufactures an important gas in the brain.

As the year comes to a close, it’s time to look back at the numerous innovations across the diabetes technology industry in 2025.

CardioKG provides a detailed view of the heart’s structure and function which dramatically improves the accuracy of predicting which genes are linked to disease and whether existing drugs could treat them.
A research team at Oregon Health & Science University has discovered a promising new drug combination that may help people with acute myeloid leukemia overcome resistance to one of the most common frontline therapies.
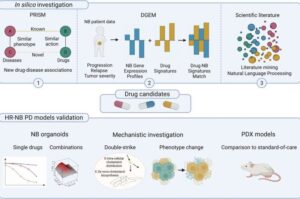
Researchers at Lund University in Sweden have identified a combination of statins and phenothiazines that is particularly promising in the treatment of the aggressive form of neuroblastoma.
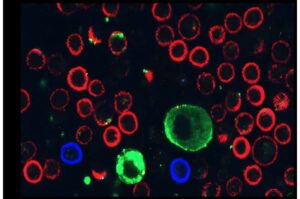
A new international study led by Prof. Carmit Levy of the Department of Human Genetics and Biochemistry at the Gray Faculty of Medical & Health Sciences at Tel Aviv University finds that melanoma cancer cells paralyze immune cells by secreting extracellular vesicles (EVs).
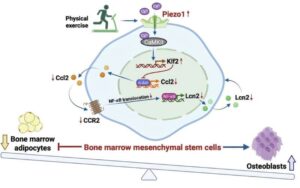
A research team from the Department of Medicine, School of Clinical Medicine, LKS Faculty of Medicine at the University of Hong Kong (HKUMed) has uncovered a key biological mechanism that explains how exercise maintains strong bones.
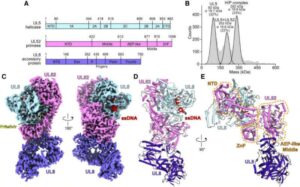
Harvard Medical School researchers have uncovered crucial insights into how an emerging class of antiviral drugs works.

Cclcium alpha-ketoglutarate (CaAKG), a safe, naturally occurring metabolite commonly studied for healthy aging, can restore key memory-related brain functions that have been disrupted in Alzheimer’s disease.