
1nhaler raises $2M for cardboard inhaler device
1nhaler announced today that it secured approximately $2 million (£1.5 million) in seed funding to support its inhaler technology.

1nhaler announced today that it secured approximately $2 million (£1.5 million) in seed funding to support its inhaler technology.
RALEIGH, N.C., Nov. 17, 2025 /PRNewswire/ — Arctx Medical, Inc. (“Arctx,” “the Company”), a clinical-stage medical device company, announced today that it has achieved two additional significant milestones:
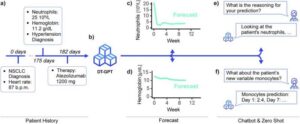
A new artificial intelligence tool that can create virtual representations of patients and predict individual health trajectories has been hailed a potential gamechanger for the clinical trial sector.

FRANKLIN LAKES, N.J., Nov. 17, 2025 /PRNewswire/ — BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced that the Conformité Européenne (CE) Marked BD Onclarity™ HPV Assay for the BD COR™ System and the BD Viper™ LT System have been accepted for the World Health Organization (WHO) list of prequalified in vitro diagnostic products, further expanding access to high-quality cervical cancer screening tools in low- and middle-income countries.
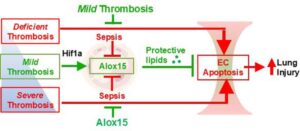
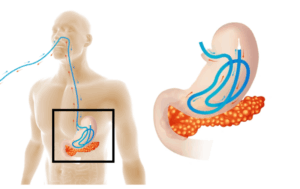
A new discovery from the lab of YouYang Zhao, Ph.D., from Stanley Manne Children’s Research Institute at Ann & Robert H. Lurie Children’s Hospital of Chicago, opens promising new directions for treatment of life-threatening lung injury caused by sepsis.
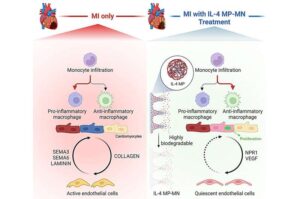
A new patch developed by Texas A&M University researcher Dr. Ke Huang may offer a way to help the heart heal after a heart attack. The patch uses a unique microneedle system to deliver a therapeutic molecule directly to damaged heart tissue, promoting repair and improving heart function without affecting the rest of the body.
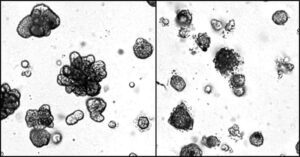
Researchers at the University of California San Diego School of Medicine have identified a promising new therapy for triple-negative breast cancer (TNBC), which is among the most aggressive and difficult-to-treat forms of the disease.
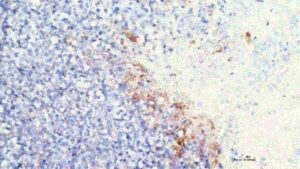
In a major step forward for cancer care, researchers at ChristianaCare’s Gene Editing Institute have shown that disabling the NRF2 gene with CRISPR technology can reverse chemotherapy resistance in lung cancer. The approach restores drug sensitivity and slows tumor growth. The findings are published in the journal Molecular Therapy Oncology.
University of Kentucky researchers have developed a new experimental model that could point the way toward more effective Alzheimer’s disease treatments by targeting one of the brain’s most important genes for risk and resilience.

The clearance is based on data that included real-world evidence from 1,120 adolescents who received treatment at US TMS centres.