New tool spots early signs of infection after breast cancer reconstruction
Researchers at Washington University School of Medicine in St. Louis have developed a new tool to detect reconstruction-related infections early, before they cause symptoms.

Researchers at Washington University School of Medicine in St. Louis have developed a new tool to detect reconstruction-related infections early, before they cause symptoms.
With this FDA approval, Hologic now offers three guideline-recommended methods: Pap testing, HPV primary testing, and co-testing.

AVENTURA, Fla., Feb. 4, 2026 /PRNewswire/ — Nuvo Intl Group Inc. today announced that pregnant moms can lease the FDA-cleared INVU wearable solution , a cloud-based pregnancy monitoring platform designed for use at home or at work, directly through their physician or healthcare provider.

TSC Life announced that it received FDA clearance for its Fluido Compact fluid warming system for pediatric use.
Testing menstrual blood for human papillomavirus (HPV) could be a “robust alternative or replacement” for current cervical cancer screening by a clinician.

For people who are at high risk of developing breast cancer, frequent screenings with ultrasound can help detect tumors early.

Northwestern University researchers have developed the first device that can continuously track a fetus’s vital signs while still in the uterus.
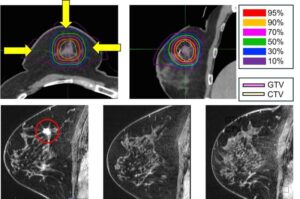
For many women with early breast cancer, surgery is effective but life-altering. New five-year data from the National Institutes for Quantum Science and Technology (QST) suggest that a precisely targeted, high-energy particle therapy may allow some patients to avoid surgery without compromising oncologic outcomes.

mOm Incubators announced today that the FDA granted 510(k) clearance for its Essential Incubator system.

Johnson & Johnson (NYSE: JNJ)+
announced that it launched the Arbrea Breast Simulator Surgeon App for Mentor in the U.S.