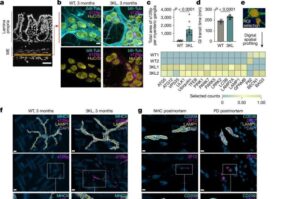
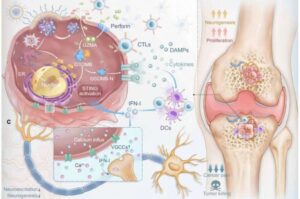
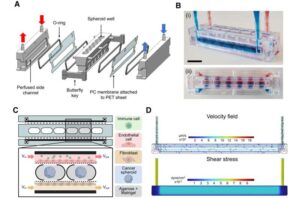
Early signs of Parkinson’s can be identified in the blood
A team led by researchers at Chalmers University of Technology, Sweden, has succeeded in identifying biomarkers for Parkinson’s disease in its earliest stages, before extensive brain damage has occurred. The biological processes leave measurable traces in the blood, but only for a limited period.