
Simple patch can make medications safer and more effective
Researchers working alongside Australian diagnostics company Nutromics developed a minimally invasive patch that tracks the antibiotic in patients every five minutes.

Researchers working alongside Australian diagnostics company Nutromics developed a minimally invasive patch that tracks the antibiotic in patients every five minutes.
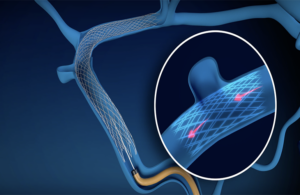
Sonorous Neurovascular received FDA breakthrough device designation for its BosSTENT device intended to treat pulsatile tinnitus, the company announced on Thursday.

More than 2,000 known CFTR mutations have been identified worldwide. The type of mutation a patient carries can alter everything from how severe their symptoms are to what drugs will work for them.
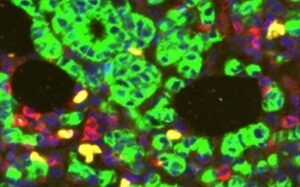
A study led by researchers at UT Southwestern, published in the Journal of Clinical Investigation, suggests that a protein secreted by immune cells within these tumors causes them to grow even in the absence of estrogen.
Researchers at Washington University School of Medicine in St. Louis have developed a new tool to detect reconstruction-related infections early, before they cause symptoms.
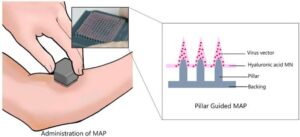
Researchers from the Institute of Industrial Science, The University of Tokyo have used 3D-printing technology to improve the viral titer of microneedle array patches, resulting in effective immunogenicity and protection against infection in mice.
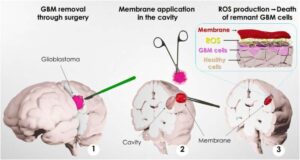
A research team led by Professor Víctor Yuste, from the Department of Biochemistry and Molecular Biology and the Institut de Neurociències de la UAB (INc-UAB), has designed and tested several bioadhesive patches that could be placed at the site where the tumor is removed during surgery, targeting any remaining cancer cells.
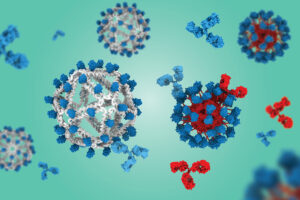
Based on a virus-like particle built with a DNA scaffold, the approach could generate broadly neutralizing antibody responses against HIV or influenza.

Next-generation vascular stents can make cardiovascular therapies minimally invasive and vascular treatments safe and less burdensome.

A new type of brain implant may have implications for both brain research and future treatments of neurological diseases such as epilepsy.