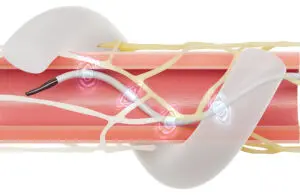
Medtronic renal denervation system wins approval in China
Medtronic (NYSE: MDT)+
today announced its Symplicity Spyral renal denervation system won National Medical Products Administration (NMPA) approval in China.

Medtronic (NYSE: MDT)+
today announced its Symplicity Spyral renal denervation system won National Medical Products Administration (NMPA) approval in China.
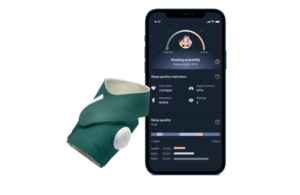
Owlet announced that it received CE mark approval for its Dream Sock device for monitoring the vital signs of infants.
WASHINGTON, May 2, 2024 /PRNewswire/ — MCRA, the leading privately held independent medical device, diagnostics, and biologics Clinical Research Organization (CRO) and advisory firm is pleased to announce its role in the successful granting of Darmiyan’s De Novo request for BrainSee by the U.S. Food and Drug Administration (FDA).

Zirconia materials now qualified for use in the production of the world’s thinnest cosmetic veneers.

Lightning Flash 2.0 features advanced computer assisted vacuum thrombectomy (CAVT) software, designed for increased efficiency and sensitivity to enhance removal of venous thrombus and treatment of pulmonary emboli (PE)

Olympus this week announced it launched the DVM-B2 Digital Video Monitor for single-use endoscopes.
Approval grants EU providers access to crucial life-saving device, indicative of the company’s global growth and ability to increase emergency and trauma care.

Monteris Medical today said its launch of the NeuroBlate NB3 FullFire makes it the smallest laser probe for brain procedures on the market.
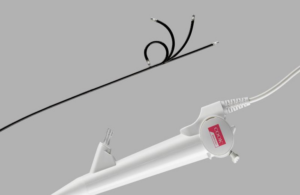
Cook Medical this week launched its Ascend Single-Use Flexible Ureteroscope in the U.S. and Canada.
MyKidneyAI Becomes the Company’s Second Innovation to Recently Receive this FDA Distinction