MedTech News
.................... by Andrew Celentano
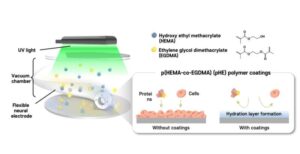
Hair-thin electrode extends brain signal recording duration three-fold
The rapid progression of an aging society has led to a sharp rise in patients with neurodegenerative diseases such as dementia and Parkinson’s disease, making it a critical issue in health care and welfare.

AI-assisted growth prediction advances orthodontics
Orthodontic treatment is most effective when timed to coincide with a child’s growth peak. Traditionally, clinicians estimate growth by examining X-ray images of the cervical vertebrae—the neck bones visible in routine dental radiographs. However, this process requires careful manual annotation of specific points on the bones, a task that is both time-consuming and prone to variation between observers.

Smartphone-based relaxation program reduces disability for emergency department migraine patients
A smartphone app for muscle relaxation significantly reduced migraine-related disability in patients visiting the emergency department, a new study shows.
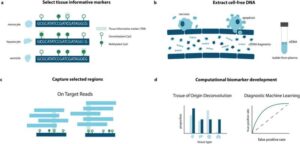
Simple blood test shows promise in detecting amyotrophic lateral sclerosis early
New research by UCLA Health has found a simple blood test could provide faster and more accurate diagnosis of ALS by measuring cell-free DNA. The noninvasive test could not only allow neurologists to rule out other neurological diseases but also detect ALS disease earlier to provide better treatment and potentially improve life expectancy.
AI analyzes world’s largest heart attack data sets—and reveals new treatment methods
A landmark international study led by the University of Zurich has shown that artificial intelligence can assess patient risk for the most common type of heart attack more accurately than existing methods. This could enable doctors to guide more personalized treatment decisions for patients.
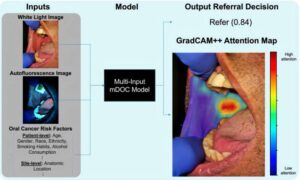
Smartphone imaging system shows promise for early oral cancer detection in dental clinics
Oral cancer remains a serious health concern, often diagnosed too late for effective treatment, even though the mouth is easily accessible for routine examination. Dentists and dental hygienists are frequently the first to spot suspicious lesions, but many lack the specialized training to distinguish between benign and potentially malignant conditions.

A pill that prints bio-ink for damaged tissue repair
EPFL researchers have demonstrated the first pill-sized bioprinter that can be swallowed and guided within the gastrointestinal tract, where it directly deposits bio-ink over damaged tissues to support repair.

OcuSciences’ eye health diagnostic instrument gains EU CE clearance
The clearance led to the deployment of the instrument across various medical facilities in the UK and Europe.
