MedTech News
.................... by Andrew Celentano
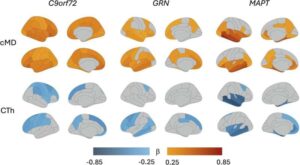
New brain imaging technique can detect early frontotemporal dementia
A new international study led by researchers at Karolinska Institutet demonstrates that it is possible to detect subtle changes in the brain and identify early signs of hereditary frontotemporal dementia using advanced brain imaging techniques. The study is published in Molecular Psychiatry.
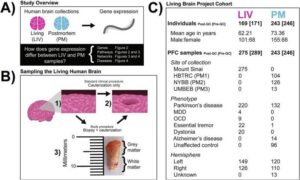
Living brain tissue reveals unique RNA and protein patterns missed in postmortem studies
Two new research papers from the Living Brain Project at Mount Sinai present what is, by several metrics, the largest investigation ever performed of the biology of the living human brain. The papers present unequivocal evidence that brain tissue from living people has a distinct molecular character, an observation that until now was missed because brain tissue from living people is rarely studied.

CAPS Medical’s PlasmaSure™ Receives FDA Breakthrough Device Designation
SANTA MONICA, Calif. and NETANYA, Israel, Oct. 15, 2025 /PRNewswire/ — CAPS Medical, a clinical-stage MedTech company developing a platform for selective tumor ablation across multiple indications, today announced that its PlasmaSure™ System has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for the treatment of low- to intermediate-risk non-muscle invasive bladder cancer (NMIBC). The PlasmaSure™ platform delivers non-thermal atmospheric plasma energy through the working channels of standard minimally invasive tools, in the case of NMIBC, through cystoscopes, enabling highly selective, tissue-preserving tumor ablation.

FAU UNVEILS WORLD’S FIRST “BENCH-TO-BEDSIDE” MRI, FOCUSED ULTRASOUND PLATFORM TO ACCELERATE RESEARCH AND TREATMENT OF NEUROLOGICAL DISORDERS
Florida Atlantic University has expanded its NeuroInnovate Center, becoming the first institution globally to integrate advanced MRI and focused ultrasound technologies into a single, unified platform for both preclinical and clinical research. This breakthrough will accelerate the development of non-invasive treatments for neurological disorders such as Alzheimer’s, Parkinson’s and more.

AI tool could make medical imaging process 90% more efficient
When doctors analyze a medical scan of an organ or area in the body, each part of the image has to be assigned an anatomical label. If the brain is under scrutiny, for instance, its different parts have to be labeled as such, pixel by pixel: cerebral cortex, brain stem, cerebellum, etc. The process, called medical image segmentation, guides diagnosis, surgery planning and research.
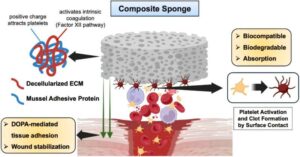
Bioadhesive sponge inspired by mussels and extracellular matrix offers new way to stop internal bleeding
Uncontrolled bleeding during surgery remains one of the deadliest medical emergencies. Injuries to internal organs such as the liver or spleen are especially dangerous because bleeding is difficult to control and often life-threatening.

Customizable finger brace toggles between stiff and flexible for easier recovery
A friend’s struggles with arthritis and the finger braces used to manage it inspired research by a Carnegie Mellon University student that could make it easier for patients to follow rehabilitation plans, speed up recovery times and help people manage chronic conditions.
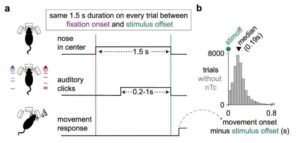
Neuroscientists—with the help of AI—can now pinpoint the moment that a brain makes a decision
For the first time, scientists can freeze-frame the exact moment an animal makes up their mind and commits to a choice—simply by looking at their brain activity.
