MedTech News
.................... by Andrew Celentano

Smartwatches detect early signs of PTSD among those watching coverage of the Oct. 7 attacks in Israel
In a three‐year study involving more than 5,000 residents of Israel before and after the mass traumatic events of October 7, 2023, those who watched extensive media coverage of the attacks were found to be more likely to develop post‐traumatic stress disorder (PTSD).

Hydrogel-based approach sustains human lymph nodes for longer, enabling more accurate studies of immune responses
A new method to keep human lymph node tissue alive and functioning outside the body for several days could give researchers a much clearer view of how our immune system responds to infections, vaccines and cancer, without the need for preclinical modeling or over-simplified laboratory models.

Millions of men could benefit from faster scan to diagnose prostate cancer
A quicker, cheaper MRI scan was just as accurate at diagnosing prostate cancer as the current 30–40 minute scan and should be rolled out to make MRI scans more accessible to men who need one, according to clinical trial results led by UCL, UCLH and the University of Birmingham.
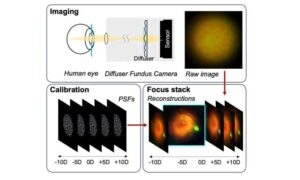
New imaging approach simplifies retina exams by enabling digital refocusing
A team of researchers from Johns Hopkins University, Boston University, and collaborators has now demonstrated a new type of fundus camera that removes one of the main challenges: focusing. Instead of relying on mechanical adjustments of a lens, the system uses a special diffuser that captures 3D light information during imaging. With this data, a computer can reconstruct and refocus images after they are taken.
Lupin Receives U.S. FDA Approval for Risperidone Long-Acting Injectable, with 180-Day CGT Exclusivity
NAPLES, Fla., Sept. 10, 2025 /PRNewswire/ — Global pharma major Lupin Limited (Lupin) (BSE: 500257) (NSE: LUPIN) (REUTERS: LUPIN.BO) (BLOOMBERG: LPCIN) today announced that it has received approval from the United States Food and Drug Administration (U.S. FDA) for its Abbreviated New Drug Application (ANDA) for Risperidone for extended-release injectable suspension, 25 mg per vial, 37.5 mg per vial, and 50 mg per vial, Single-Dose Vials.

Abbott reports first commercial Tendyne mitral valve implant in the U.S.
An Abbott (NYSE: ABT)+
official announced on social media that doctors completed the first commercial implant of Tendyne in the U.S.
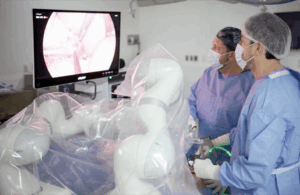
Levita Magnetics reports first AI-guided autonomous gall bladder surgery with MARS system
Levita Magnetics announced today that surgeons used its MARS platform to perform the world’s first gall bladder surgery featuring an autonomous surgical camera.
Pulse Biosciences wins FDA approval for AF study using nsPFA technology
The trial will evaluate the effectiveness of the nsPFA cardiac surgical system during concurrent surgical procedures.
