MedTech News
.................... by Andrew Celentano

Temperature-sensing patch invented for early breast cancer detection
A Ph.D. student at the University of Bristol has developed a convenient and cost-effective wearable patch to measure subtle temperature changes across the breast, which could in future be used to detect potential abnormalities and cancerous tumors.

Nasal mask support improves breathing in preterm babies in clinical trial
Using a nasal mask instead of a traditional face mask to support very premature babies at birth can significantly reduce the need for rescue breathing and escalation of care, a world-first trial led by researchers at Monash University’s School of Clinical Sciences at Monash Health, in collaboration with Hudson Institute of Medical Research and Monash Children’s Hospital (Monash Health), has shown.
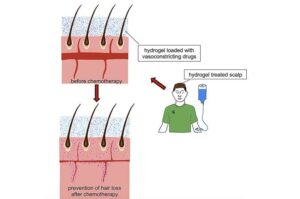
Shampoo-like gel could help chemo patients keep their hair
Cancer fighters know that losing their hair is often part of the battle, but Michigan State University researchers have developed a shampoo-like gel that has been tested in animal models and could protect hair from falling out during chemotherapy treatment.

Don’t sweat it: New device detects sweat biomarker at minimal perspiration rate
Available on demand, in abundance and containing multiple biomarkers, sweat is an increasingly appealing medium for monitoring health, according to researchers at Penn State. But not everyone—especially critically ill patients—can build up enough sweat to provide a robust enough sample for current analysis techniques.
Single antibody may be responsible for life-threatening reaction to common blood thinner
Researchers at McMaster University have discovered that a rare but dangerous reaction to a widely used blood thinner is caused by a single antibody—overturning decades of medical misunderstanding and opening the door to more precise ways of diagnosing and treating this medical complication.
Foldax introduces TRIA mitral valve in India
Dolphin Life Science India handles the local production of the TRIA mitral valve.

Q&A: Daye launches at-home hormone testing service across the UK
Daye has just launched a comprehensive at-home hormone testing service across the UK, and Med-Tech Insights spoke to Valentina Milanova, founder & CEO of Daye to find out more.

Tasso and SheMed provide at-home blood testing to UK women for personalised GLP-1 treatments
Tasso, a specialist in patient-centric blood collection, and SheMed, a UK-based women’s healthcare company, have teamed up to deliver safer, more personalised weight loss treatments through convenient at-home diagnostics.
