MedTech News
.................... by Andrew Celentano
BrightHeart Secures Third FDA Clearance and PCCP Approval for Integrated Fetal Heart Monitoring Solution
First-of-its-kind platform offers real-time fetal heart documentation and congenital heart disease detection, revolutionizing prenatal care and diagnosis.
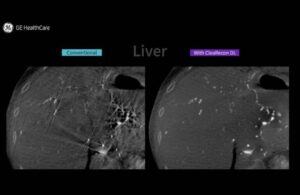
GE HealthCare launches AI-based 3D reconstruction solution
GE HealthCare (Nasdaq: GEHC) today announced the launch of its CleaRecon DL technology for enhancing CBCT imaging.
EndoQuest Robotics completes first cases in pivotal surgical robot trial
EndoQuest Robotics announced today that investigators completed the first procedures of the PARADIGM pivotal clinical trial of its surgical robot
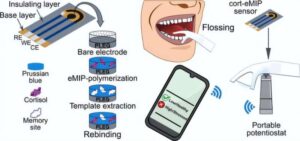
A new dental flosser enables at-home stress monitoring
Now, in ACS Applied Materials & Interfaces, researchers report a dental floss pick with a built-in sensor that could monitor stress as part of a daily routine. The device, which accurately senses levels of the stress hormone cortisol in minutes, could help users recognize when it’s time to get help.

Go-Pen ApS wins FDA nod for user-filled insulin pen
Go-Pen ApS announced recently that it received FDA 510(k) clearance for its Go-Pen cost-effective, user-filled insulin pen.

Drug injection device wins MIT $100K Competition
CoFlo Medical’s low-cost device could administer advanced biologic treatments more quickly to people with cancers, autoimmune diseases, and more.

Breastfeeding device measures babies’ milk intake in real time
Soft, comfortable wearable device takes the guesswork out of breastfeeding
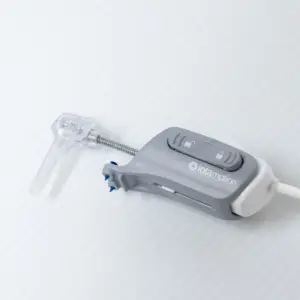
iotaMotion Launches First Robotic-Assisted Cochlear Implant Procedure Outside the U.S
Swiss Clinical Trial Marks Key Step in Global Expansion of Precision Hearing Implant Technology
