MedTech News
.................... by Andrew Celentano

Johnson & Johnson MedTech launches mobile simulator for breast procedures
Johnson & Johnson (NYSE: JNJ)+
announced that it launched the Arbrea Breast Simulator Surgeon App for Mentor in the U.S.
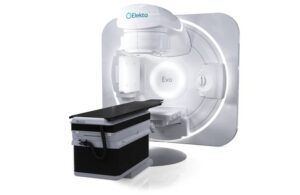
Elekta gets FDA nod for AI-powered linear accelerator for radiology
Elekta announced today that its Evo CT linear accelerator (linac) received FDA 510(k) clearance, making it available to U.S. radiation oncology professionals.
Finding new cell markers to track the most aggressive breast cancer in blood
Of all the types of breast cancer, triple negative breast cancer (TNBC) is the most aggressive and lacks specific therapies. TNBC is also more likely to metastasize, or travel through the bloodstream to spread to other organs, which causes most of breast cancer-related deaths each year.
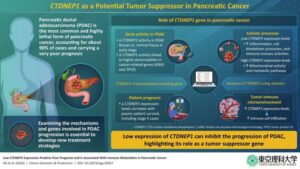
Potential tumor-suppressing gene identified in pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer and begins in the cells lining the pancreatic duct. Accounting for more than 90% of all pancreatic cancers, PDAC is extremely difficult to treat and has a very high mortality rate.

Basivertebral nerve ablation provides early, sustained chronic low back pain relief
Chronic low back pain significantly affects quality of life for millions of people worldwide. Back pain makes it difficult to perform everyday tasks and is among the leading reasons why patients see their doctor.
Reprogrammed skin cells shed light on HIV-related cognitive impairment
Using participant skin cells reprogrammed into neurons, Weill Cornell Medicine researchers have identified genetic signatures associated with HIV infection that may contribute to the cognitive impairment that often occurs in people living with HIV, even when the virus is controlled.
New blood test shows extent of brain injury after stroke—and reveals treatment effects
Strokes are a medical emergency, yet imaging can capture only snapshots of how brain damage develops in the hours and days that follow. For many other organs, blood tests can indicate acute injury, but until now the brain has lacked a comparable marker. Researchers at LMU University Hospital and international partners report that a new blood biomarker, brain-derived tau (BD-tau), can track the extent of brain injury after ischemic stroke over time.
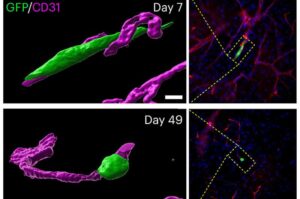
Dormant cancer cells can change shape to survive immune system attack
Cancer cells that have broken away from a primary tumor can lurk in the body for years in a dormant state, evading immune defenders and biding their time until conditions are ripe for establishing a new tumor elsewhere in the body, a process known as metastasis.
