MedTech News
.................... by Andrew Celentano
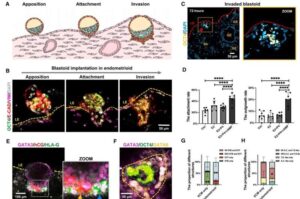
How a miniature womb on a chip can help women struggling to conceive
A team of scientists from China has successfully created a miniature womb on a chip that mimics the complex environment of the human uterus. The research offers a new way to study the exact moment an embryo attaches to a mother’s body.

LEADOPTIK Announces FDA Clearance of the LIA™ for Lung Biopsy Procedures
SAN JOSE, Calif., Jan. 15, 2026 /PRNewswire/ — LEADOPTIK, Inc. today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its Last Inch Assessment™ (LIA) system, the world’s first system to use silicon photonics imaging technology and software designed to improve the accuracy of lung biopsy procedures.

Neurolief receives FDA PMA Approval for First At-Home Brain Neuromodulation Therapy for Adults Whose Depression Was Not Adequately Improved by Antidepressants
CORAL SPRINGS, Fla., Jan. 12, 2026 /PRNewswire/ — Neurolief Inc., a medical device company focused on neuromodulation therapies for neuropsychiatric conditions, today announced that the U.S. Food and Drug Administration (FDA) has approved Proliv™Rx, the first prescription, physician-directed, at-home brain neuromodulation therapy as an adjunctive treatment for adults with Major Depressive Disorder (MDD) who failed to achieve satisfactory improvement from at least one previous antidepressant medication.
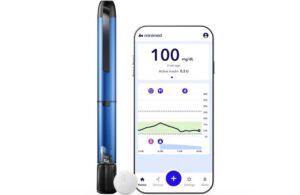
Medtronic wins FDA clearance for MiniMed Go app for InPen smart insulin pen
Medtronic (NYSE: MDT)+
announced today that the FDA granted 510(k) clearance for its MiniMed Go app for multiple daily injections (MDI).
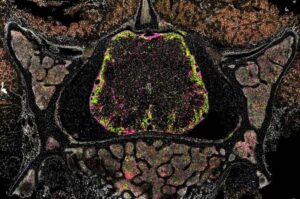
New insight into the immune signals driving inflammation in multiple sclerosis
Multiple sclerosis (MS) is a chronic neurological disease characterized by nerve damage and consequent impairments in vision, movement, balance and mental function. In MS, the immune system mistakenly starts attacking myelin, the protective sheath that surrounds axons (i.e., nerve fibers) in the brain, spinal cord and optic nerves.

‘Unique’ AI-powered headset can predict epilepsy seizures
A “unique” AI-powered headset that can predict epileptic seizures minutes before they occur has been developed by scientists in Scotland.

Genetic study uncovers unknown causes of blindness
Researchers from Radboud University Medical Center and University of Basel have discovered new genetic causes of inherited blindness.
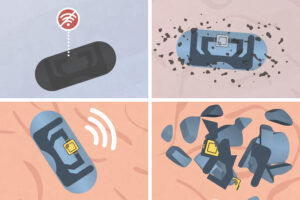
Pills that communicate from the stomach could improve medication adherence
MIT engineers designed capsules with biodegradable radio frequency antennas that can reveal when the pill has been swallowed.
