MedTech News
.................... by Andrew Celentano

Fecal transplant capsules show promising results in clinical trials for multiple types of cancer
Fecal microbiota transplants (FMT) can dramatically improve cancer treatment, suggest two groundbreaking studies published in the Nature Medicine journal.
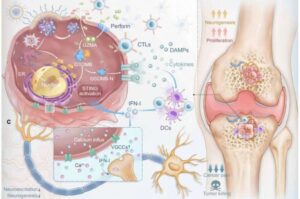
New nanotherapy eases bone metastasis pain by disrupting tumor-nerve crosstalk
A new nano-sized drug carrier that finds bone tumors and releases treatment exactly where it’s needed is here to improve the precision and comfort of cancer therapy.

Glaukos gets FDA nod for iDose TR re-administration
Glaukos (NYSE:GKOS) announced today that the FDA approved a new drug application (NDA) labeling supplement for its iDose TR system.

Long COVID brain fog far more common in US than India, other nations
Large study of patients in U.S., Colombia, Nigeria and India finds symptom burden highest in high-income countries
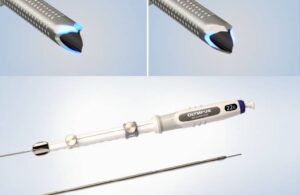
Olympus launches single-use fine needle biopsy device in US
Olympus today announced the U.S. launch of its most advanced single-use fine needle biopsy device.
FDA clears eMurmur’s next-gen AI heart murmur detection software
eMurmur has received FDA 510(k) clearance for its next-generation heart murmur detection software, eMurmur Heart AI (2.2).
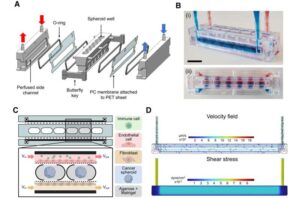
3D chip platform enables animal-free testing in cancer research
Cancer research laboratory tests can now be done using micro-physiological systems mimicking human physiology.
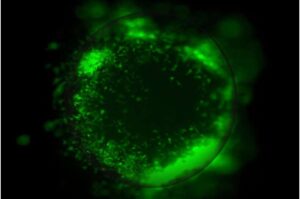
Lab-grown organoids reveal how glioblastoma outsmarts treatment
UCLA scientists have developed advanced miniature 3D tumor organoid models that make it possible to study glioblastoma tumors in a setting that closely mirrors the human brain, shedding light on how the aggressive cancer interacts with surrounding brain cells and the immune system to become more invasive and resistant to therapy.
