MedTech News
.................... by Andrew Celentano

Scientists map the human genome in 4D
Study is a landmark effort to understand how DNA’s physical structure influences human biology
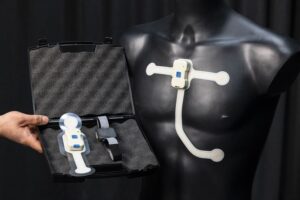
Wearable sensors assist in developing safer and better home dialysis – HUS and VTT start testing with patients
ESPOO, Finland, Jan. 8, 2026 /PRNewswire/ — HUS Helsinki University Hospital and VTT are starting a field test of wearable sensors to develop home dialysis. A total of 36 volunteer patients will use the sensors that enable monitoring of treatment effectivity. Making home dialysis safer and better would improve life quality and save society millions of euros annually.

Saluda Medical Receives Regulatory Approval for EVA™ Sensing Technology in Europe with recognition in Australia
MINNEAPOLIS, Jan. 8, 2026 /PRNewswire/ — Saluda Medical, Inc. (ASX:SLD, “Saluda” or the “Company”), a commercial-stage medical device company focused on developing treatments for chronic neurological conditions using its novel closed-loop neuromodulation platform, announced that, as expected, its next-generation EVA™ Sensing Technology has now received CE certification for commercialization in Europe with recognition of this approval in Australia. This follows FDA approval of EVA in December 2024.
Short-circuiting pancreatic cancer: A potential RNA therapy
Cold Spring Harbor Laboratory (CSHL) Professor Adrian Krainer’s lab discovered how the protein SRSF1 along with AURKA and MYC, jumpstart PDAC tumor development.
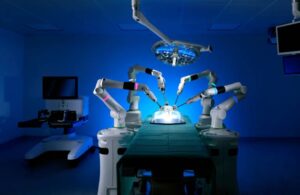
CMR Surgical wins CE mark for Versius in pediatric procedures
CMR Surgical announced today that it received CE mark and U.K. approval for its Versius surgical robot in pediatric surgery.

Medtronic launches small-diameter defibrillation lead
Medtronic this week launched its OmniaSecure defibrillation lead in the U.S., describing it as the world’s smallest.
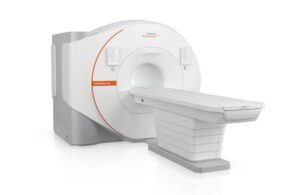
Siemens Healthineers wins FDA nod for helium-free MRI system
Siemens Healthineers announced today that it received FDA clearance for its 70cm bore Magnetom Flow magnetic resonance imaging (MRI) platform.

Stem cell therapy for stroke shows how cells find their way in the brain
Some parts of our bodies bounce back from injury in fairly short order. The outer protective layer of the eye—called the cornea—can heal from minor scratches within a single day. The brain is not one of these fast-healing tissues or organs.
